Toxoplasma IgM antibody immunoblotting kit and preparation method thereof
A technology of immunoblotting and toxoplasma gondii, which is applied in the field of kits, can solve the problems of high price and achieve the effect of low cost, wide application, simple and fast detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of described toxoplasma gondii IgM antibody immunoblotting kit comprises the following steps:
[0034]1) Preparation of SAG1 (P30), SAG2 (P22), ROP2 and GRA7 Toxoplasma gondii recombinant antigens
[0035] Using gene cloning technology, PCR amplified the DNA encoding the T. gondii antigen, and inserted it into Escherichia coli to express it, and obtained the T. gondii recombinant antigens SAG1 (P30), SAG2 (P22), ROP2 and GRA7.
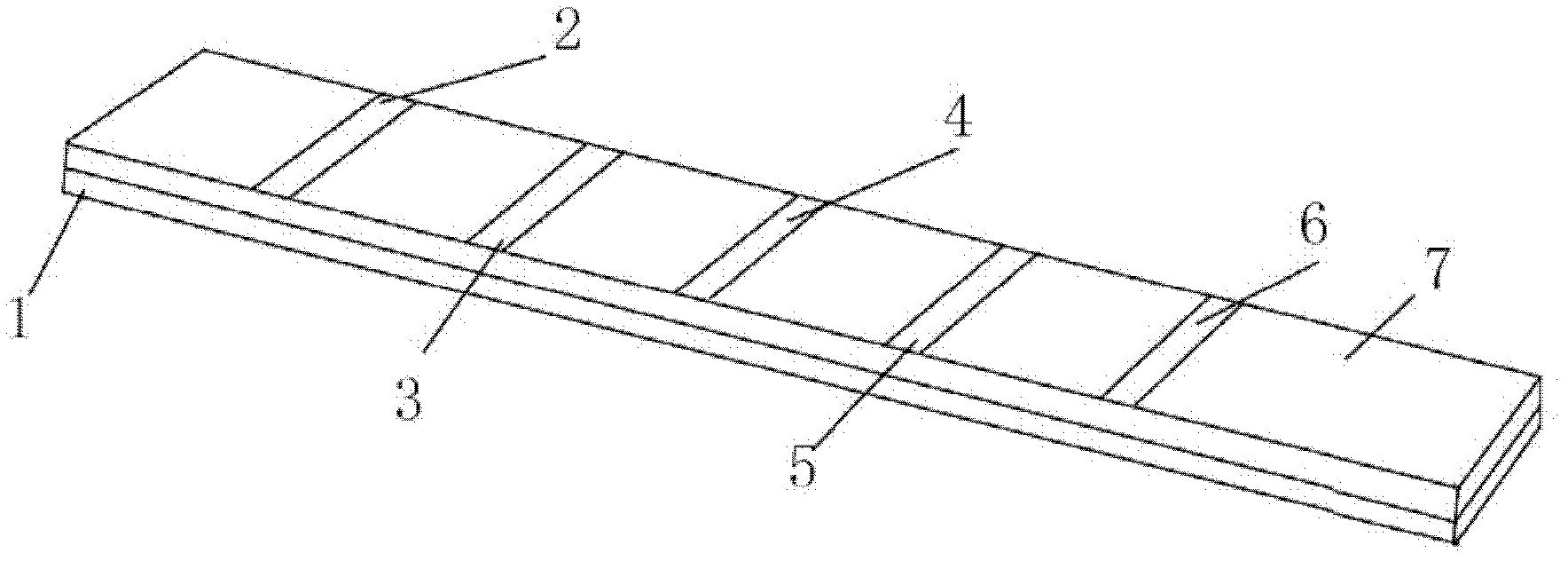
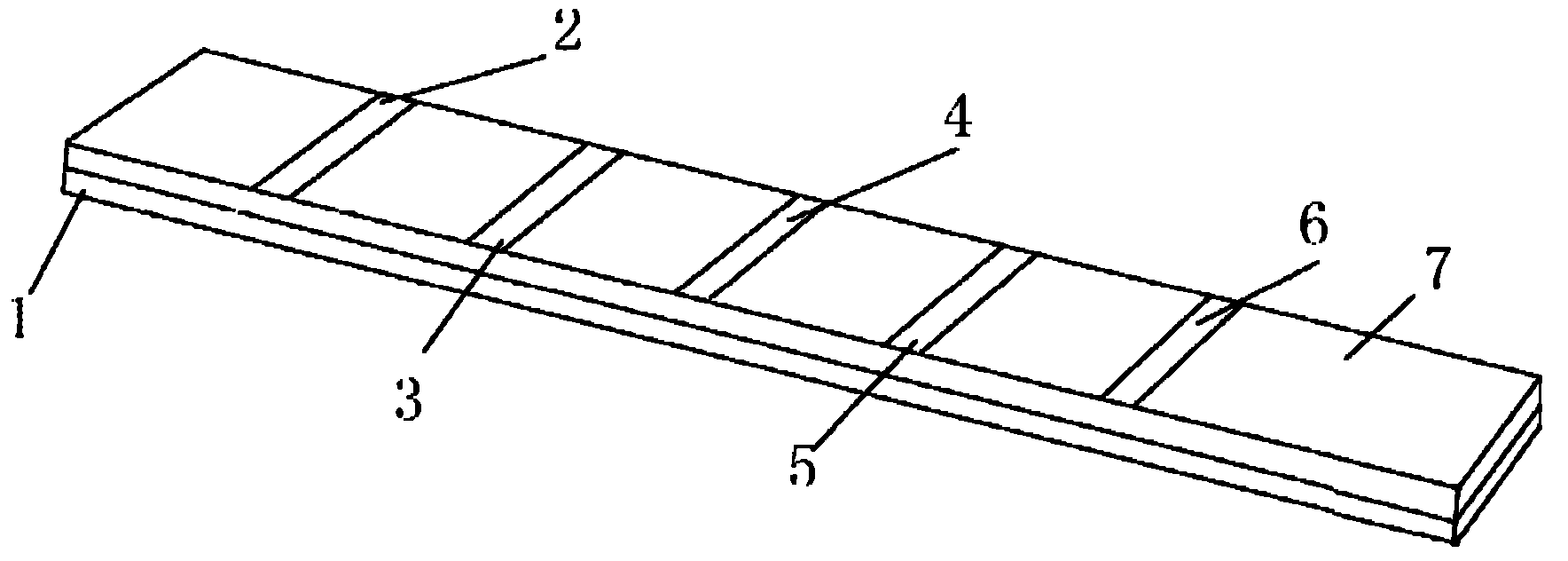
[0036] 2) Spotting on nitrocellulose membrane
[0037] SAG1 (P30), SAG2 (P22), ROP2 and GRA7 Toxoplasma recombinant antigens were coated on the nitrocellulose membrane IgM detection line, and human IgM antibody was coated on the control line, and dried in the air.
[0038] 3) Preparation of anti-human IgM specific fragment μ chain monoclonal antibody
[0039] Balb / c mice were immunized with human IgM-specific fragment μ chain as antigen, and hybridoma cell lines stably secreting anti-human IgM monoclonal antibody were sc...
Embodiment 1
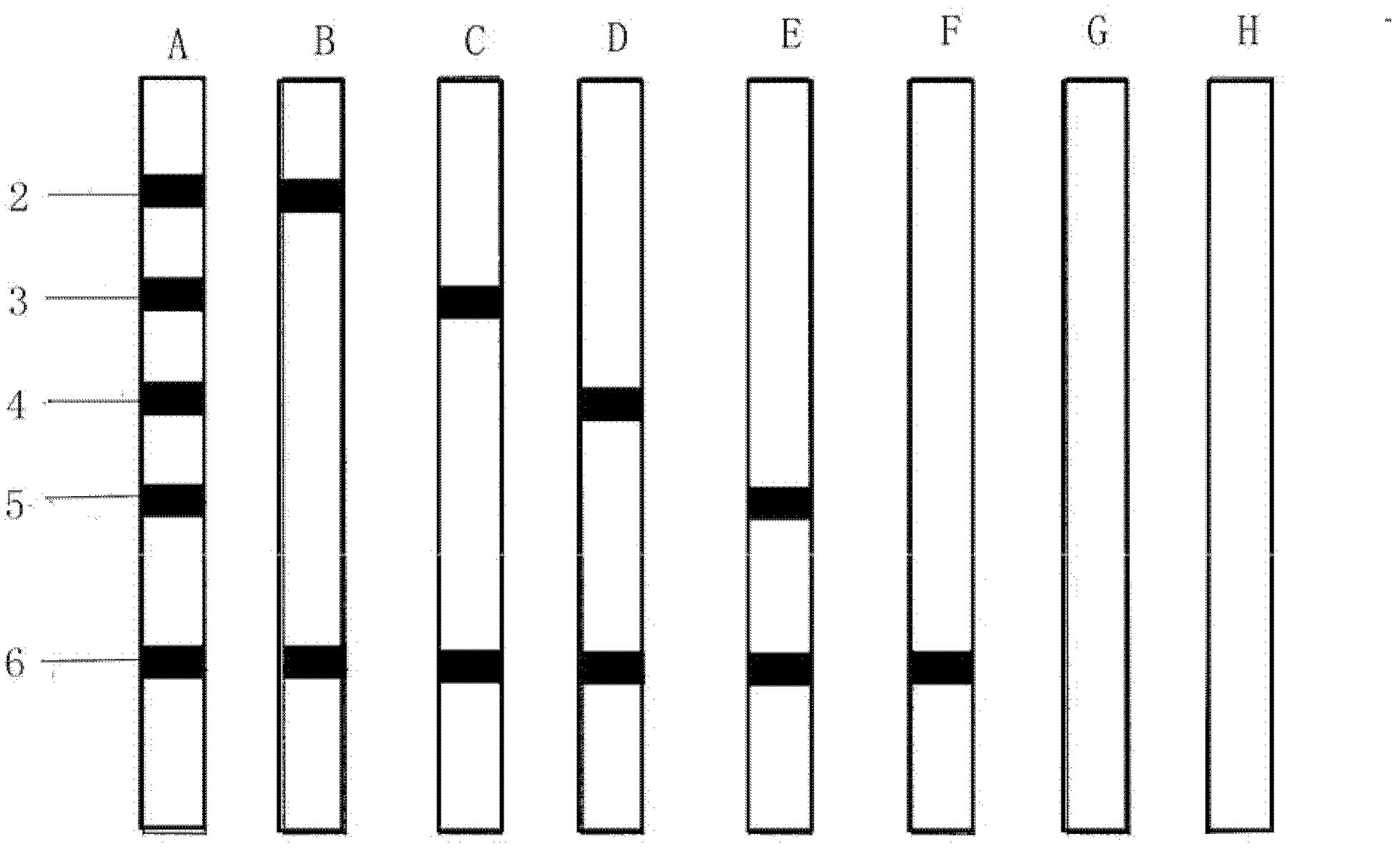
[0065] Paste the nitrocellulose membrane on the surface of the carrier plate, and the Toxoplasma gondii IgM antibody detection line and the control line are successively arranged on the nitrocellulose membrane; the Toxoplasma gondii IgM antibody detection line is coated with Toxoplasma gondii recombinant antigen SAG1 (P30), SAG2 (P22 ), ROP2 and GRA7, coated with human IgM antibody at the control line, cut into strips with a strip cutter, and assembled with enzyme-labeled anti-human μ chain monoclonal antibody and its substrate to form Toxoplasma gondii IgM antibody immunoblotting reagent box.
[0066] 1) The sample was diluted 1:50 with 0.01mol / LPBS buffer.
[0067] 2) Blocking: 5% skimmed milk powder (prepared with 0.01 mol / L PBST buffer) was used as the blocking solution, and incubated at room temperature (18-25° C.) on a rocking table for 30 min.
[0068] 3) Serum incubation: take out the required membrane strips, put them into the incubation tank, and number them. Add 1...
Embodiment 2
[0076] Similar to Example 1, the difference is that the nitrocellulose membrane detection line only consists of SAG2 (P22), ROP2 and GRA7 Toxoplasma recombinant antigens, and does not contain SAG1 (P30) Toxoplasma recombinant antigens. Result judgment is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com