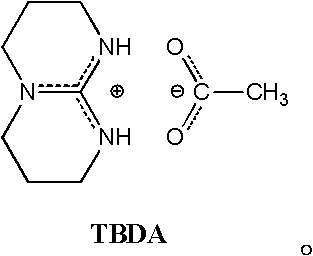
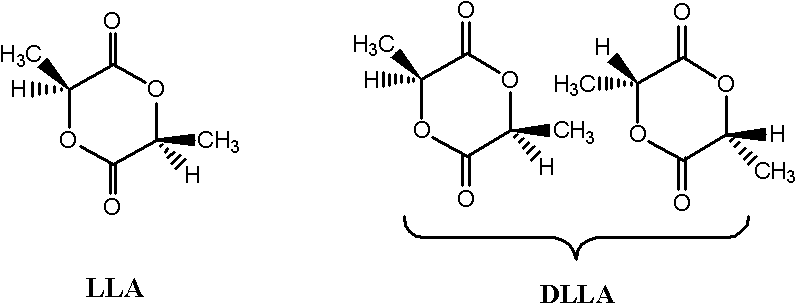
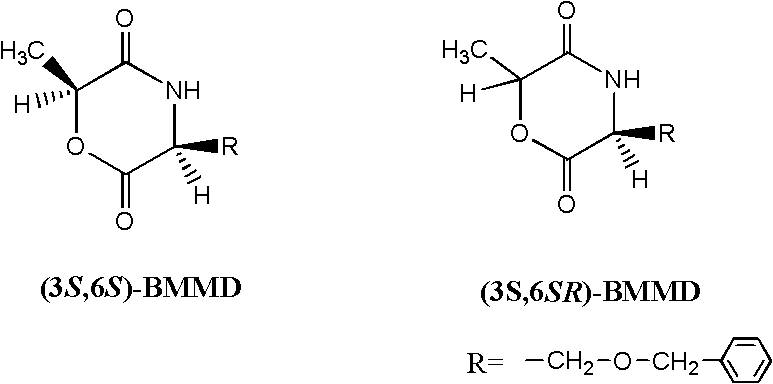
Process for synthesizing lactic acid-serine copolymer by catalyzing and carrying out ring-opening copolymerization on acetate bicyclo guanidine
A serine copolymer, bicyclic guanidine catalyzed lactide technology, applied in non-active ingredients of medical preparations, pharmaceutical formulations, pharmaceutical science and other directions, can solve the problems of poor catalytic effect, potential safety hazards, etc., to achieve a high degree of biological safety , the effect of reducing production costs and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1——bicyclic guanidine acetate catalyzes L-lactide and (3S, 6S)-serine lactate morpholine diketone ring-opening copolymerization (the reaction conditions of this example and the following examples are to bicyclic guanidine acetate catalysis D, L- Ring-opening copolymerization of lactide and (3S)-serine lactate morpholine dione is also applicable, the same below)
[0033] Add monomer L-lactide 1.000g (6.900mmol) and (3S, 6S)-serine lactate morpholine dione 0.0352g (0.142mmol) in the reaction kettle, control L-lactide and (3S, 6S) -serine morpholine diketone mol ratio is: 98 / 2; Add catalyst bicyclic guanidine acetate 0.0141g (0.071mmol), control the molar weight sum of comonomer lactide and serine morpholine diketone to be the same as that of catalyst bicyclic guanidine acetate The molar ratio is: 100 / 1. After three times of vacuum-filling with argon, the mixture was heated to 130° C. for 3 minutes under vacuum. Then the reaction kettle was moved to 100° C., a...
Embodiment 2
[0036] Example 2—Bicyclic guanidine acetate catalyzed ring-opening copolymerization of L-lactide and (3S, 6S)-serine lactate morpholine dione
[0037] Add monomer L-lactide 1.000g (6.900mmol) and (3S, 6S)-serine lactate morpholine dione 0.1103g (0.443mmol) in the reaction kettle, control L-lactide and (3S, 6S) -serine morpholine diketone mol ratio is: 94 / 6; Add catalyst bicycloguanidine acetate 0.0147g (0.074mmol), control the molar weight sum of comonomer lactide and serine morpholine diketone to be the same as that of catalyst bicycloguanidine acetate The molar ratio is: 100 / 1. After three times of vacuum-filling with argon, the mixture was heated to 140° C. for 5 minutes under vacuum. Then the reaction kettle was moved to 130° C., and the reaction was continued for 20 minutes.
[0038] 20ml of dichloromethane was used to dissolve the polymer in a pressure test tube. Then 5 ml of triethylsilane, 0.1 ml of triethylamine and 0.15 g of palladium dichloride were added. React...
Embodiment 3
[0040] Example 3—Bicyclic guanidine acetate catalyzed ring-opening copolymerization of L-lactide and (3S, 6S)-serine lactate morpholine dione
[0041] Add monomer L-lactide 1.000g (6.900mmol) and (3S, 6S)-serine lactate morpholine dione 0.1921g (0.772mmol) in the reaction kettle, control L-lactide and (3S, 6S) -serine morpholine diketone mol ratio is: 90 / 10; Add catalyst bicyclic guanidine acetate 0.0154g (0.077mmol), control the molar weight sum of comonomer lactide and serine morpholine diketone to be the same as that of catalyst bicyclic guanidine acetate The molar ratio is: 100 / 1. After three vacuum-argon operations, the mixture was heated to 150° C. for 4 minutes under vacuum. Then the reaction kettle was moved to 120° C., and the reaction was continued for 30 minutes.
[0042] 20ml of dichloromethane was used to dissolve the polymer in a pressure test tube. Then 5 ml of triethylsilane, 0.1 ml of triethylamine and 0.15 g of palladium dichloride were added. Reaction at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com