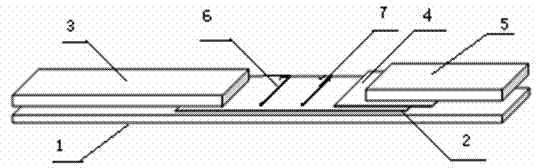
Contagious caprine plueropneumonia antibody detection test strips, and preparation method thereof
A technology for antibody detection and pleuropneumonia, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as oversensitivity, false positives, and poor sensitivity, and achieve the effect of reducing economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of colloidal gold: Colloidal gold was prepared by trisodium citrate reduction method.
[0027] 2. Preparation of colloidal gold-labeled polysaccharide antigen: firstly, use 0.1mol / L KCO 3 Adjust the pH value to 6.0; then determine the optimal amount of colloidal gold and the polysaccharide antigen to be labeled by visual inspection, and obtain the optimal labeling amount of 1mg / 100ml, add Mycoplasma goat pneumoniae sub A polysaccharide antigen, let it stand for 30min; centrifuge the above colloidal gold at 2500r / min for 30min, remove the precipitate, and obtain a supernatant; centrifuge the supernatant at 10000r / min for 30min, discard the supernatant to collect the precipitate, and the precipitate Suspend in gold colloid buffer that is 1 / 10 times the volume of initial colloidal gold, and store at 4°C; gold colloid buffer is phosphate buffer containing 5‰ sucrose, 1% BSA, 0.5‰ PEG20000, 0.5‰ Tween-20 , concentration 0.02mol / L, pH7.4.
[0028] 3. Assembly o...
Embodiment 2
[0033] 1. Preparation of colloidal gold: Colloidal gold was prepared by trisodium citrate reduction method;
[0034] 2. Preparation of colloidal gold-labeled polysaccharide antigen: first, use 0.1mol / L KCO 3 Adjust the pH value to 7.0; then determine the optimal amount of colloidal gold and the polysaccharide antigen to be labeled by visual inspection, and obtain the optimal labeling amount of 3.4mg / 100ml, add Mycoplasma capricosum to the colloidal gold solution at 3.4mg / 100ml Pneumonia subspecies polysaccharide antigen, let it stand for 30min; centrifuge the above colloidal gold at 2500r / min for 30min, remove the precipitate, and obtain the supernatant; centrifuge the supernatant at 10000r / min for 30min, discard the supernatant to collect the precipitate, and Suspend the precipitate in gold colloidal buffer that is 1 / 10 times the volume of the initial colloidal gold, and store at 4°C; the gold colloid buffer is phosphate containing 15‰ sucrose, 1% BSA, 1‰ PEG20000, 1‰ Tween-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com