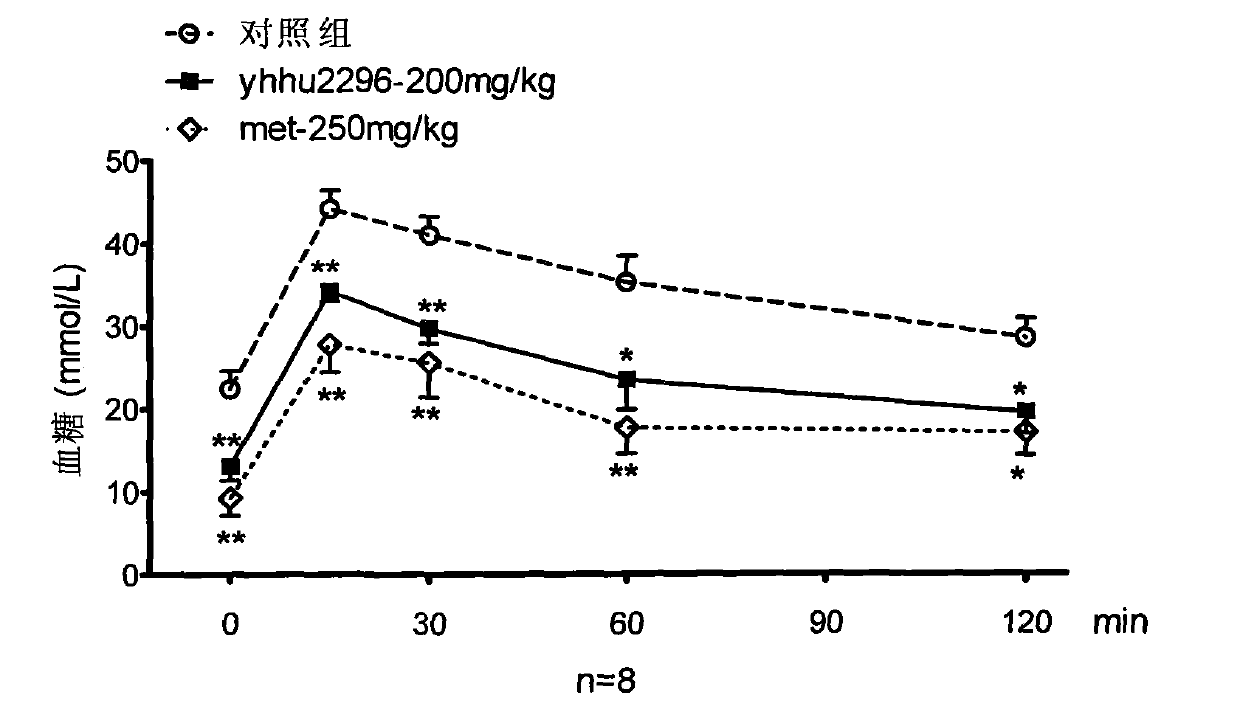
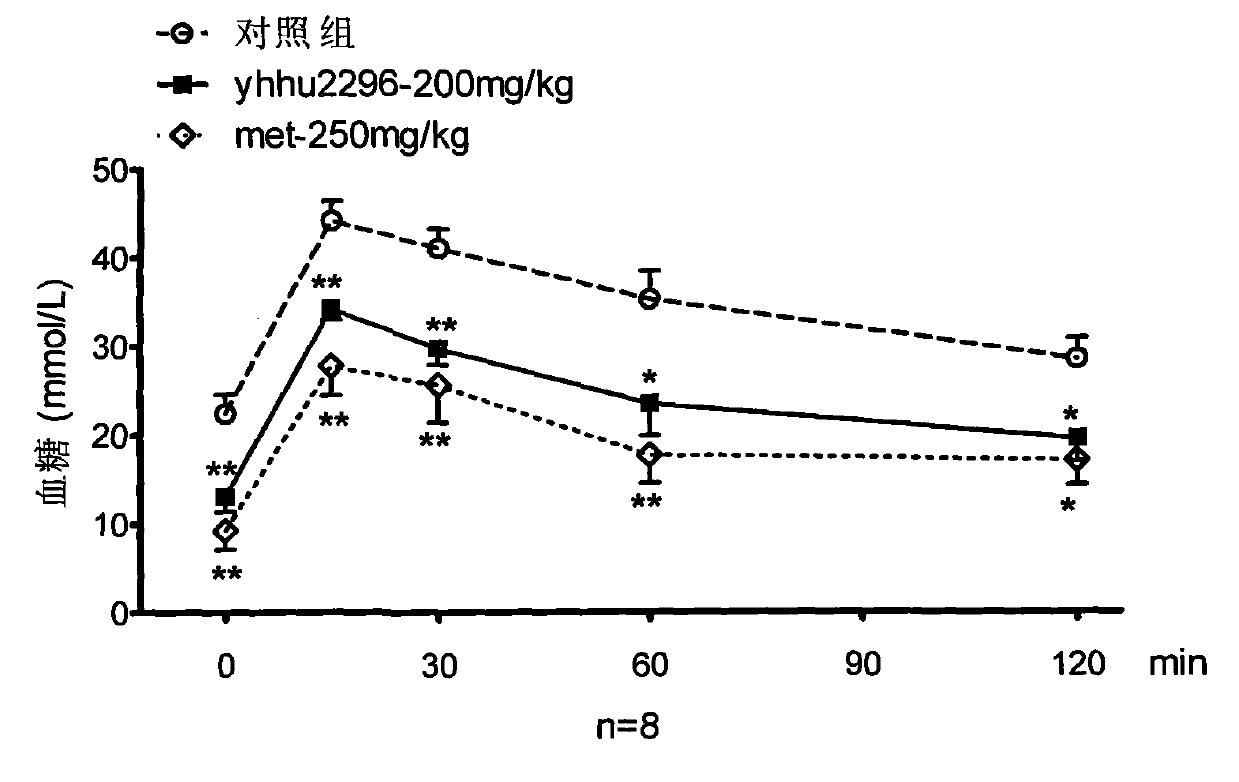
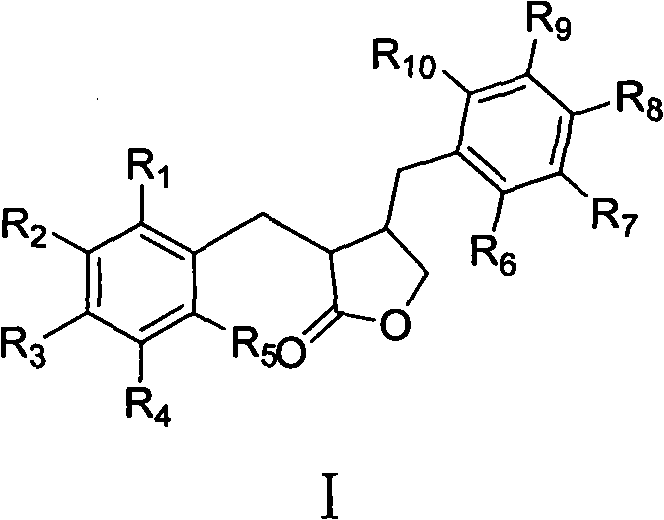
Application of dihydrofuran-2-ketone compounds in preparing medicament for resisting diabetes mellitus and glucose and lipid metabolism
A technology of ketone compounds and dihydrofuran, which is applied in the field of pharmacy, can solve the problems of ineffective hypoglycemic effect, high toxicity, and many side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of 4-(3,4-dimethoxybenzyl)-3-(4-methoxybenzyl)dihydrofuran-2(3H)-one (compound 10a)
[0052]
[0053] 1-1: Preparation of Compound 6a
[0054] 67.6g (0.602mol) potassium tert-butoxide is dissolved in 600ml tert-butanol, dimethyl succinate and 3,4-dimethoxybenzaldehyde (0.602mol) are mixed and dissolved in a small amount of tert-butanol, and then Add it dropwise to the solution of potassium tert-butoxide, control the temperature of the reaction system not higher than 40°C, stir at room temperature for 30 minutes, pour the mixture into water (1000ml), wash with isopropyl ether (500ml), and wash the aqueous phase with concentrated Acidify with hydrochloric acid, extract with ethyl acetate (1000ml), take the ethyl acetate layer, wash with water, and wash with saturated sodium chloride solution once each, dry over anhydrous sodium sulfate, dry the solvent by rotary evaporation to obtain a yellow solid, add 100ml of ether, and oscillate ultrasonicall...
Embodiment 2
[0065] Example 2: Preparation of 4-(3,4-dimethoxybenzyl)-3-(4-hydroxyl-3-methoxybenzyl)dihydrofuran-2(3H)-one (compound 10b)
[0066]
[0067] 2-1: Preparation of Compound 7b
[0068] Dissolve compound 11b (1.88g, 12.3mmol) in 10ml methanol, add K 2 CO 3 (2.1g, 14.8mmol), refluxed for 2h, monitored by TLC, filtered and concentrated after the reaction was complete, the residue was dissolved in chloroform, washed twice with 20ml of water, dried over anhydrous sodium sulfate, and concentrated to obtain compound 7b.
[0069] 2-2: Preparation of compound 10b'
[0070] Compound 10b' was prepared in a similar manner as shown in Example 1.
[0071] 10b': 1 HNMR (300MHz, CDCl 3 ): δ7.25-7.48(m, 5H), 6.79(d, 1H, J=8.2Hz), 6.74(d, 1H, J=9.4Hz), 6.71(d, 1H, J=2.4Hz), 6.59 (dd, 1H, J=1.9, 8.1Hz), 6.52(dd, 1H, J=1.9, 8.1Hz), 6.47(d, 1H, J=1.9Hz), 5.13(s, 2H), 4.07-4.18( m, 1H), 3.80-3.92(m, 1H), 3.85(s, 3H), 3.84(s, 3H), 3.80(s, 3H), 2.90-3.00(m, 2H), 2.42-2.68(m, 4H) ).
[0072...
Embodiment 3
[0074] Example 3: Preparation of 4-(3,4-dimethoxybenzyl)-3-(3,4-dihydroxybenzyl)dihydrofuran-2(3H)-one (compound 10c)
[0075]
[0076] Compound 10c was prepared in a similar manner to Compound 10a in Example 1, except that the corresponding starting reactants were used.
[0077] 10c: 1HNMR (300MHz, CDCl3): δ6.41-6.78(m, 6H), 3.97(dd, J=13.12, J=8.92, 2H), 3.82(s, 3H), 3.81(s, 3H), 3.77 (s, 3H), 3.74 (s, 3H), 3.00-2.85 (m, 2H), 2.67-2.43 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com