Method for obtaining high-purity zinc by electrolyzing zinc chloride
A zinc chloride, high-purity technology, applied in the direction of photography technology, equipment, photography auxiliary technology, etc., to achieve the effect of solving post-processing problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Put 80g of ammonium chloride and 100g of sodium chloride into a beaker respectively, stir and dissolve with deionized water.
[0032] 2. Dissolve 160 grams of zinc chloride in the above conductive saline solution.
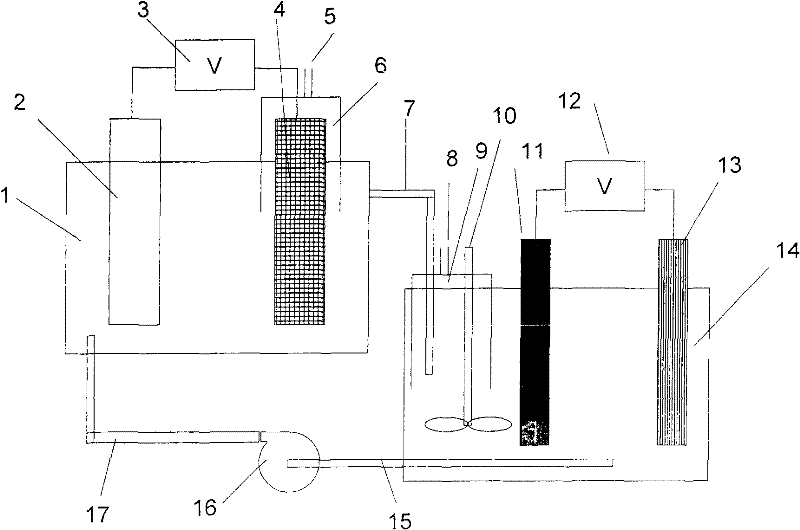
[0033] 3. Use such as figure 1 In the shown electrolysis system device, the solution in the beaker is transferred to a large volume cylinder of 1L and then poured into a 1L plexiglass buffer tank 14 .
[0034]4. Use a zinc sheet with a Zn99.99% content as an anode of 30×40mm, and a 30×40mm polished pure aluminum sheet as a cathode, and turn on a 0.02A direct current to electrolyze the above electrolyte for 1 hour.
[0035] 5. Use a peristaltic pump to inject the electrolyte after electrolytic treatment with a small current into a 300mL glass electrolytic cell 1, use a RuTi-coated DSA anode of 40×60mm, a polished aluminum cathode of 40×60mm, and control the electrode distance to 4cm, and pass 2A DC current for electrolysis .
[0036] 6. After 1 hour of ...
Embodiment 2
[0054] 1. Put 80g of ammonium chloride and 100g of sodium chloride into a beaker respectively, stir and dissolve with deionized water.
[0055] 2. Dissolve 160 grams of zinc chloride in the above conductive saline solution.
[0056] 3. Use such as figure 1 In the shown electrolysis system device, the solution in the beaker is transferred to a large volume cylinder of 1L and then poured into a 1L plexiglass buffer tank 14 .
[0057] 4. Use a zinc sheet with a Zn99.99% content as an anode of 30×40mm, and a 30×40mm polished pure aluminum sheet as a cathode, and turn on a 0.02A direct current to electrolyze the above electrolyte for 1 hour.
[0058] 5. Use a peristaltic pump to inject the electrolytic solution after low-current electrolysis treatment into the 300mL glass electrolytic cell 1, use RuTi-coated DSA anode 40×60mm, use Zn99.99% zinc sheet cathode 40×60mm, and control the electrode distance to 4cm , Through 2A DC current electrolysis.
[0059] 6. After 1 hour of elect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com