Dentifrice composition
A composition and dentifrice technology, applied in the fields of medical science, dentistry, oral care, etc., can solve the problems of not obtaining satisfactory results, reduce pathogenic bacteria, etc., and achieve good use feeling, high bactericidal power, smoothness Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
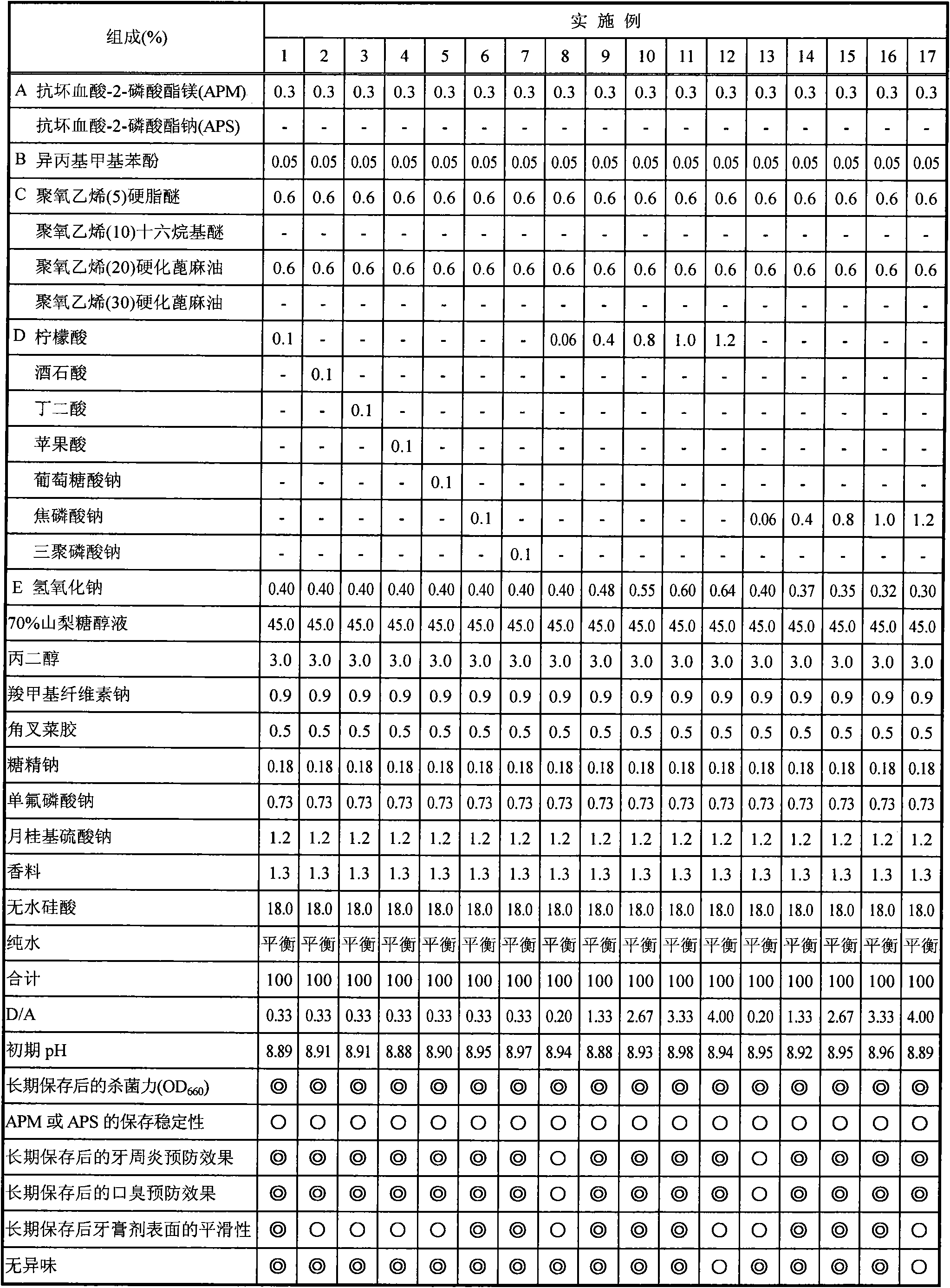
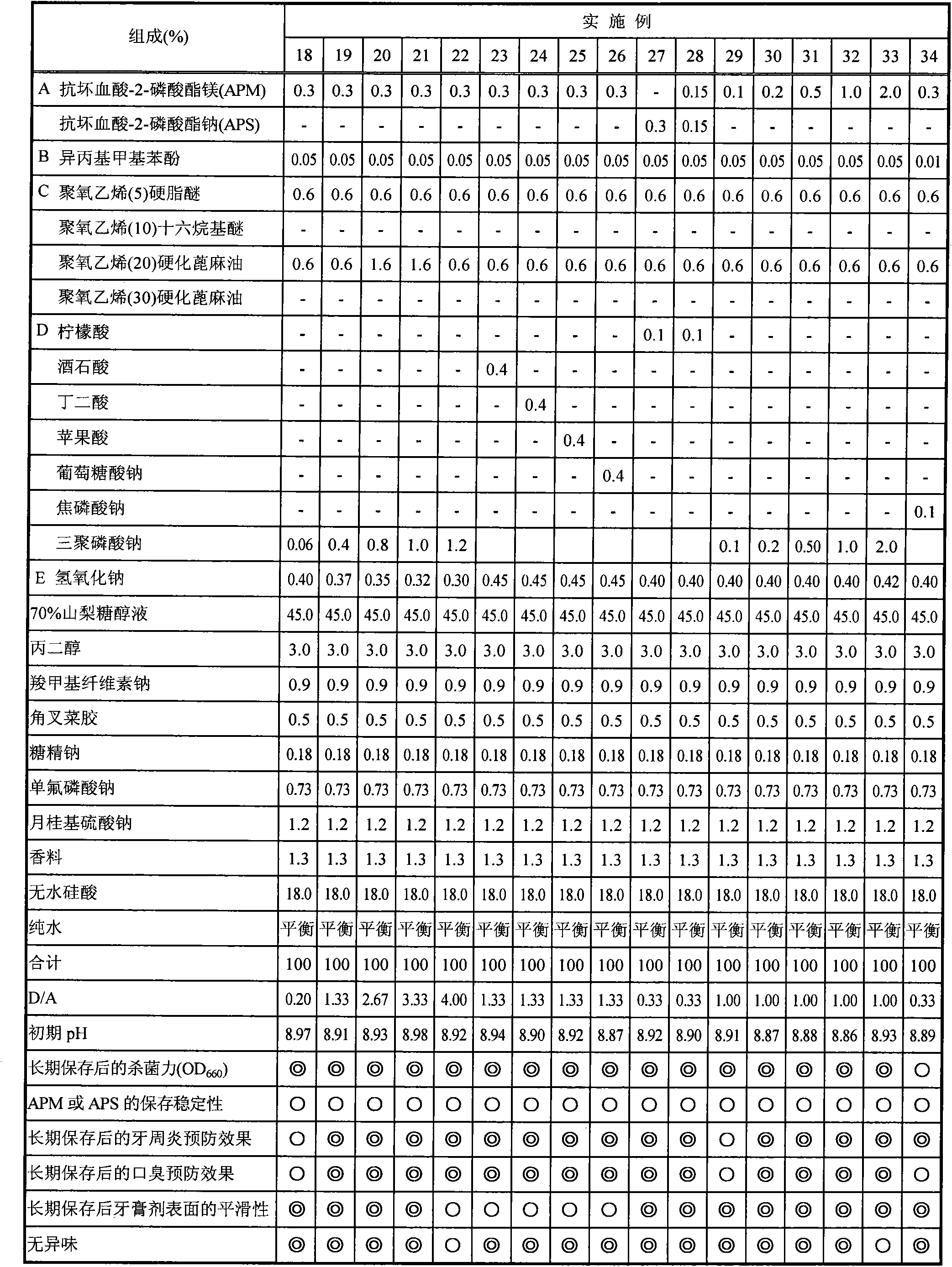
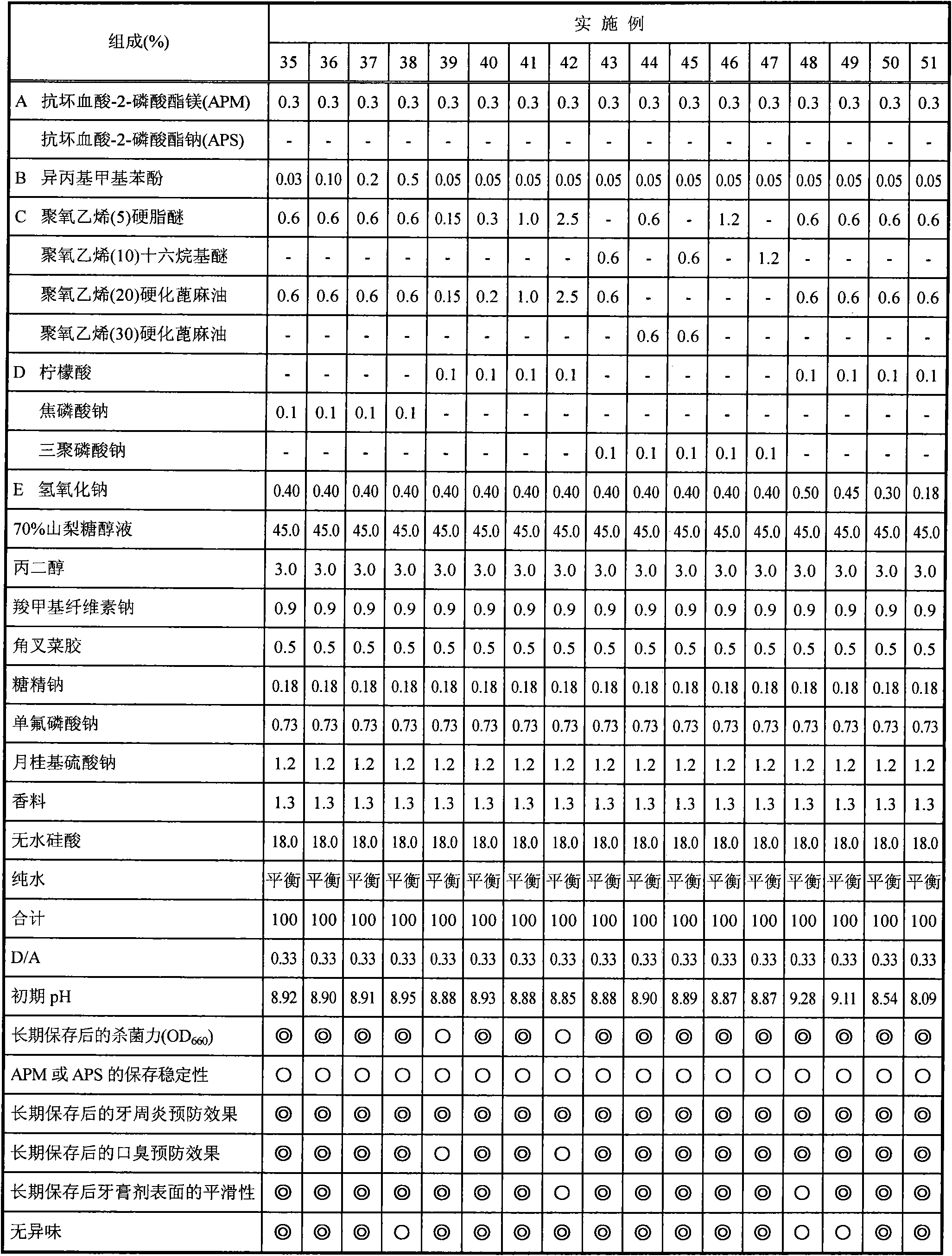
[0063] Hereinafter, the present invention will be specifically described by way of experimental examples, examples, comparative examples, and formulation examples, but the present invention is not limited to the following examples. In addition, in the following examples, any of the % symbols means mass % unless otherwise specified.
[0064] In addition, the dentifrice composition of the composition shown in Tables 1-4 was prepared according to the following manufacturing method.
[0065] The method of manufacture of the dentifrice composition:
[0066] (1) Mix and dissolve water-soluble ingredients (except binder and propylene glycol) in pure water at room temperature to prepare Phase A.
[0067] (2) Disperse the binder in propylene glycol at room temperature to prepare Phase B.
[0068] (3) Add and mix phase B into phase A under stirring to prepare phase C.
[0069] (4) Use a 6L kneader (manufactured by Ishiyama Works) at room temperature to mix ingredients other than wate...
experiment example 1
[0079] [Experimental example 1] Evaluation of bactericidal power after storage at 40°C for 6 months
[0080] After preparing the dentifrice composition (toothpaste) shown in Tables 1-4, it preserved in 40 degreeC thermostat for 6 months. After storage, place until it reaches normal temperature, measure 10 g of each dentifrice composition, add human oral saliva (30 mL), stir and centrifuge (10,000 rpm, 20 min), and take the supernatant as the sample stock solution. Disperse the pre-cultured oral bacteria dispersion (Fusobacterium nucleatum) in human oral saliva, and make the turbidity (OD 660 ) adjusted to 2mL of the solution at 1 o'clock, after acting for 30 seconds, 50 μL was taken as a sample for bactericidal evaluation, and the bactericidal effect was evaluated according to the following method.
[0081] Bactericidal power evaluation:
[0082] Each bactericidal activity evaluation sample solution (50 μL) was added to THB liquid medium (4 mL), and after incubating at 37° C...
experiment example 2
[0089] [Experimental Example 2] Storage stability of magnesium ascorbyl-2-phosphate (hereinafter abbreviated as APM), sodium ascorbyl-2-phosphate (hereinafter abbreviated as APS) or sodium ascorbate after storage at 40°C for 6 months
[0090] After preparing the dentifrice compositions shown in Tables 1 to 4, they were stored in -5°C and 40°C thermostats for 6 months. Samples stored at -5°C were used as non-decomposed controls. After this was left to return to normal temperature, it was used for the evaluation of the storage stability of APM, APS, or sodium ascorbate, and averaged according to the following method. All the reagents were manufactured by Kanto Chemical Co., Ltd.
[0091] Quantification of APM or APS:
[0092] Weigh 0.1g of each dentifrice composition, add 10mmol / L phosphate buffer solution (1.5mmol / L potassium dihydrogen phosphate, 23.5mmol / L dipotassium hydrogen phosphate, pH 8.0), and stir vigorously with an oscillator for 20 minutes , and set the volume to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com