Selective hydrogenation reduction method for aromatic nitro compound
A technology for aromatic nitro compounds, which is applied in the field of selective hydrogenation reduction of aromatic nitro compounds, can solve the problems of restricting the wide application of catalysts and complex preparation, and achieve the effects of strong practicability, mild reduction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
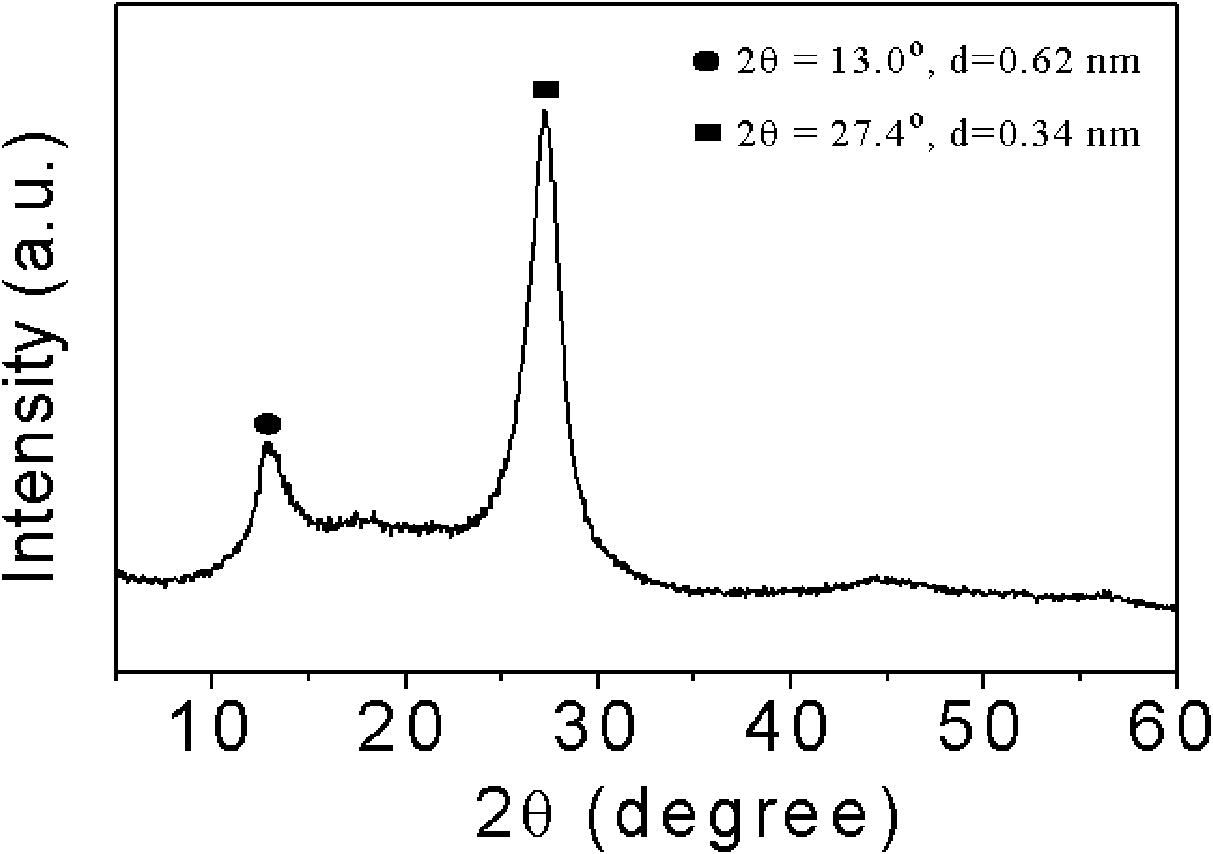
[0023] Take 3g of cyanamide, dissolve it in 3g of water, add 407.5g of Ludox HS, raise the temperature to 80°C to remove water, and obtain a white solid, heat up to 550°C at 2.5°C / min to polymerize for 4h, cool to room temperature and use 4M / L Ammonium bifluoride solution removes the template agent, and after drying, a yellow mesoporous carbon nitride material is obtained. There are two peaks in XRD analysis, and 2θ=27.4° is the (002) peak of the material, representing that the distance between the material layers is 0.34nm , 2θ=13.0° is the (100) peak of the material, which represents the regularity of the layered structure of the material, and the specific surface area of the material is 200m 2 / g.
[0024] Add 0.2g of mesoporous carbon nitride material into a 250mL beaker, add 100mL of deionized water, ultrasonically shake for 30 minutes, and then add 4ml of PdCl with a concentration of 0.056mmol / ml 2 Aqueous solution, ultrasonic vibration for 30 minutes, add 5mg NaBH 4...
Embodiment 2
[0027] The Pdmpg-C prepared by embodiment 1 3 N 4 Put 15mg of catalyst into a 50ml three-neck round-bottom flask, add 2ml of water, take 1mmol of the substrate p-amidonitrobenzene and put it into the round-bottom flask, replace the air in the round-bottom flask with hydrogen for three times, and connect the round-bottom flask to a hydrogen bag at atmospheric pressure , reacted at room temperature for 7 hours. After the reaction is completed, filter and rinse with ethanol three times. Gas chromatography analysis shows that p-nitrobenzonitrile is completely converted into p-amidoaniline, and the conversion rate and selectivity are both greater than 99%.
Embodiment 3
[0029] The Pdmpg-C prepared by embodiment 1 3 N 4 Catalyst 15mg is packed into 50ml three-neck round-bottomed flask, add 2ml water, get substrate p-nitrobenzaldehyde 1mmol and add into round-bottomed flask, replace the air in the round-bottomed flask with hydrogen for three times, connect the round-bottomed flask with the atmospheric pressure hydrogen bag, The reaction was carried out at room temperature for 7 hours. After the reaction is completed, filter and rinse with ethanol three times. Gas chromatography analysis shows that p-nitrobenzonitrile is completely converted into p-aminobenzaldehyde, and the conversion rate and selectivity are both greater than 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com