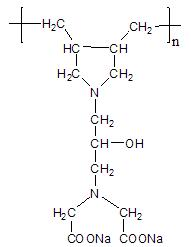
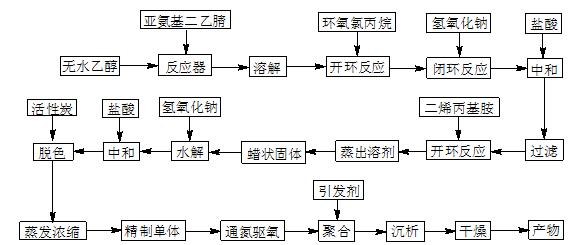
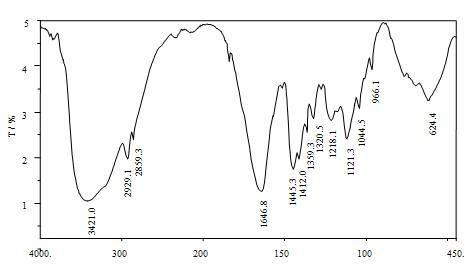
Macromolecule chelating agent and preparation method thereof
A polymer chelating agent, light yellow technology, applied in the field of polymer chelating agent and its preparation, to achieve the effect of expanding the application range, improving the use effect, and reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) According to the volume ratio of absolute ethanol and epichlorohydrin as 10:1, first add 158mL of absolute ethanol to a 500mL three-necked bottle with a reflux condenser and a mechanical stirrer, and then add 17.1g of imino di Acetonitrile was stirred until dissolved, then 15.8mL of epichlorohydrin was added dropwise, and reacted at 40°C for 6h;
[0030] (2) Keep the temperature at 40°C, add 14.4mL of NaOH solution with a mass percentage concentration of 45% to the solution in step (1) dropwise within 2h, continue to react for 1h after the dropwise addition, and then add 6.7mL of 6mol / L hydrochloric acid and excess NaOH, filtered to remove NaCl, then added 22.4mL diallylamine to the filtrate and continued the reaction at 70°C for 4h, distilled off the solvent to obtain 41.7g light yellow waxy solid;
[0031] (3) Add the solid obtained in step (2) to 365 mL of NaOH solution with a mass percent concentration of 4.5%, heat up to 95°C for hydrolysis for 0.5 h, add 12 mL...
Embodiment 2
[0033] (1) According to the volume ratio of absolute ethanol and epichlorohydrin as 15:1, first add 237mL of absolute ethanol to a 500mL three-necked bottle with a reflux condenser and a mechanical stirrer, and then add 18.1g of imino di Acetonitrile was stirred until dissolved, then 15.8mL epichlorohydrin was added dropwise, and reacted at 50°C for 5h;
[0034] (2) Keep the temperature at 50°C, add 13.3mL NaOH solution with a mass percentage concentration of 48% to the solution in step (1) dropwise within 2h, continue to react for 1h after the dropwise addition, and then add 6.7mL of 6mol / L hydrochloric acid and excess NaOH, filtered to remove NaCl, then added 23.6mL diallylamine to the filtrate to continue the reaction at 60°C for 4.5h, distilled off the solvent to obtain 43.6g light yellow waxy solid;
[0035] (3) Add the solid obtained in step (2) to 345 mL of NaOH solution with a mass percent concentration of 5%, heat up to 95° C. for hydrolysis for 1 h, add 12.7 mL of 6m...
Embodiment 3
[0037] (1) According to the volume ratio of absolute ethanol and epichlorohydrin as 20:1, first add 316mL of absolute ethanol to a 500mL three-necked bottle with a reflux condenser and a mechanical stirrer, then add 19g of iminodiacetonitrile Stir until dissolved, then add 15.8mL of epichlorohydrin dropwise, and react at 60°C for 4h;
[0038] (2) Keep the temperature at 60°C, add 12.6mL of 50% NaOH solution dropwise to the solution in step (1) within 2h, continue to react for 1h after the dropwise addition, and then add 6.7mL of 6mol / L hydrochloric acid and excess NaOH, filtered to remove NaCl, then added 24.9mL diallylamine to the filtrate and continued the reaction at 70°C for 4h, distilled off the solvent to obtain 45.6g light yellow waxy solid;
[0039] (3) Add the solid obtained in step (2) to 364 mL of NaOH solution with a mass percent concentration of 5%, heat up to 95° C. for hydrolysis for 1 h, add 13.3 mL of 6 mol / L hydrochloric acid to neutralize excess NaOH after c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com