Method for improving hydrogen storage property of lithium borohydride
A lithium borohydride and performance technology, which is applied in the field of improving the hydrogen storage performance of lithium borohydride, can solve problems such as low rate, and achieve the effects of improving hydrogen storage performance, improving diffusion capacity and improving hydrogen storage performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
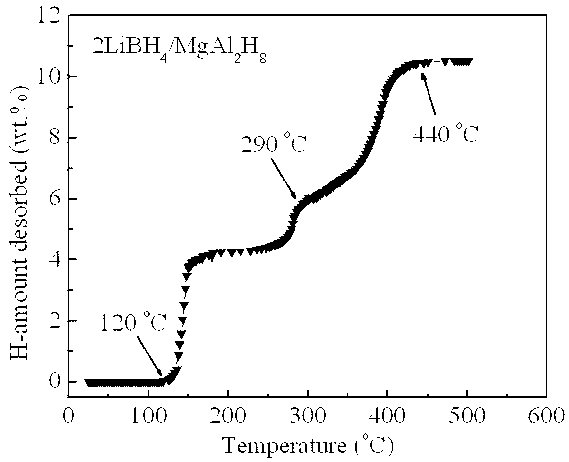
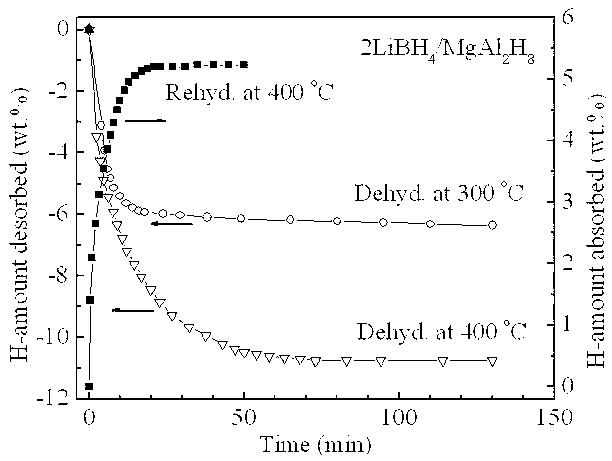
[0019] Example 1: In a glove box filled with argon, LiBH was weighed according to a molar ratio of 2:1 4 and MgAl 2 h 8 The two raw material powders were poured into a stainless steel ball mill tank with a volume of 250 ml, and mechanically mixed with a planetary ball mill for 2 h at a ball-to-material ratio of 20:1, a speed of 400 rpm, and a vacuum. At a heating rate of 3°C / min, for 2LiBH 4 / MgAl 2 h 8 The mixed powders were heat treated and their thermal hydrogen release properties were measured. Such as figure 1 Shown, MgAl 2 h 8 Complete its decomposition process in the temperature range of 120 ~ 290 ° C (X-ray diffraction analysis shows that the decomposition product is Mg 2 Al 3 and Al), in MgAl 2 h 8 Under the catalysis of the decomposition products, LiBH 4 Hydrogen release starts at 290°C and ends at 440°C, 2LiBH 4 / MgAl 2 h 8 The total hydrogen release of the mixed powder is as high as 10.5 wt.%. figure 2 2LiBH 4 / MgAl 2 h 8 The first hydrogen des...
Embodiment 2
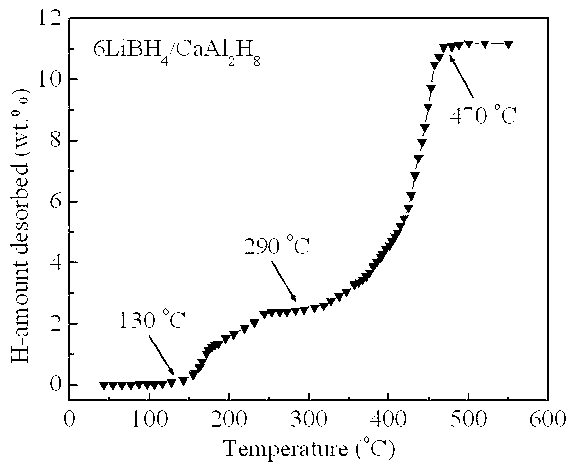
[0020] Example 2: In a glove box filled with argon, LiBH was weighed according to a molar ratio of 6:1 4 and CaAl 2 h 8 The two raw material powders were poured into a stainless steel ball mill tank with a volume of 250 ml, and mechanically mixed with a planetary ball mill for 2 h at a ball-to-material ratio of 20:1, a speed of 400 rpm, and argon protection. At a heating rate of 3°C / min, for 6LiBH 4 / CaAl 2 h 8 The mixed powders were heat treated and their thermal hydrogen release properties were measured. Such as image 3 Shown, CaAl 2 h 8 Complete its decomposition process in the temperature range of 130 ~ 250 ° C (X-ray diffraction analysis shows that the decomposition product is CaH 2 and Al), in CaAl 2 h 8 Under the catalysis of the decomposition products, LiBH 4 Hydrogen release starts at 290°C and ends at 470°C, 6LiBH 4 / CaAl 2 h 8 The total hydrogen release of the mixed powder is as high as 11.2 wt.%. Figure 4 6LiBH 4 / CaAl 2 h 8 Kinetic curves of hy...
Embodiment 3
[0021] Example 3: In a glove box filled with argon, LiBH was weighed according to a molar ratio of 4:1 4 and Sr 2 H 7 The two raw material powders were poured into a stainless steel ball mill tank with a volume of 100 ml, and mechanically mixed with a planetary ball mill for 4 h at a ball-to-material ratio of 15:1, a speed of 400 rpm, and a vacuum. At a heating rate of 3°C / min, for 4LiBH 4 / Sr 2 H 7 The mixed powders were heat treated and their thermal hydrogen release properties were measured. Such as Figure 5 Shown, Sr 2 H 7 Complete its decomposition process in the temperature range of 160 ~ 270 ° C (X-ray diffraction analysis shows that the decomposition product is SrAl 4 and SrH 2 ), at Sr 2 H 7 Under the catalysis of the decomposition products, LiBH 4 The dehydrogenation starts at 300°C, and the dehydrogenation process can be basically completed at 460°C, and the hydrogen desorption amount is greater than 5.0 wt.%. At the same time, 4LiBH 4 / Sr 2 H 7 The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com