Method for preparing 4-felbinac through rearrangement reaction
A felbinac and rearrangement reaction technology, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield, difficult industrialization, difficult removal of impurities, high toxicity, etc., and achieve the effects of easy industrial production, simple operation, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
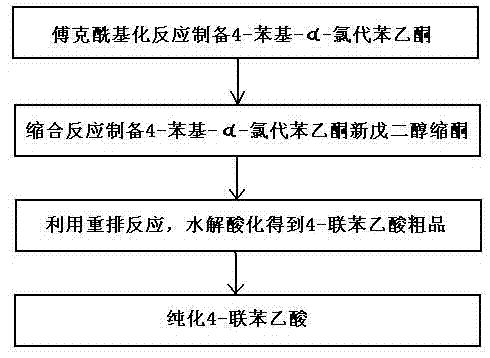
[0046] A method utilizing rearrangement reaction to prepare 4-biphenylacetic acid, the steps comprising:
[0047] (1) Preparation of 4-phenyl-a-chloroacetophenone by Friedel-Crafts acylation reaction
[0048] Dissolve 30.8 grams of biphenyl (0.2moL) and 24.6 grams of chloroacetyl chloride (0.22moL) in 50mL of dichloromethane, and add them to the dropping funnel for later use; meanwhile, add 32 grams of aluminum trichloride (0.24mol) and 150mL Add dichloromethane into the three-necked flask with mechanical stirring, and cool it to below 0°C under rapid stirring, then drop the mixture of biphenyl and chloroacetyl chloride in the dropping funnel into the three-necked flask, Complete the addition within 2 hours, continue to stir at below 0°C for 1 hour, and detect with gas chromatography until the biphenyl content is less than 1%;
[0049] After the reaction is complete, pour the reaction solution into 200 g of ice water and stir rapidly to complete the hydrolysis; several layers...
Embodiment 2
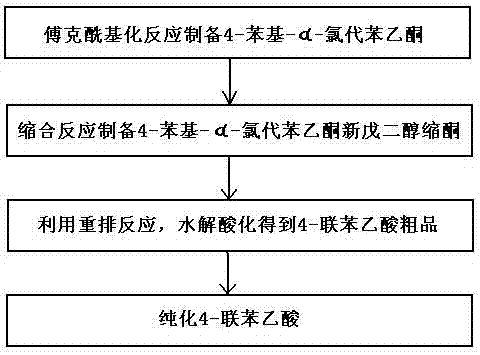
[0059] A method utilizing rearrangement reaction to prepare 4-biphenylacetic acid, the steps comprising:
[0060] (1) Preparation of 4-phenyl-a-chloroacetophenone by Friedel-Crafts acylation reaction
[0061] Dissolve 92.4 grams of biphenyl (0.6moL) and 73.8 grams of chloroacetyl chloride (0.66moL) in 100mL of dichloromethane, and add to the dropping funnel for later use; at the same time, 96 grams of aluminum trichloride (0.72mol) and 250mL Add dichloromethane into a three-necked flask with mechanical stirring, and cool it to below -10°C under rapid stirring, then drop the mixture of biphenyl and chloroacetyl chloride in the dropping funnel into the three-necked flask, Add within 2 hours, continue to stir at -10°C for 2 hours, and detect with gas chromatography until the biphenyl content is less than 1%;
[0062] After the reaction is complete, pour the reaction liquid into 400 grams of ice water, and stir rapidly to complete the hydrolysis; separate and collect several laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com