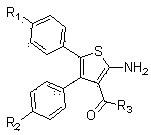
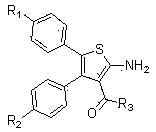
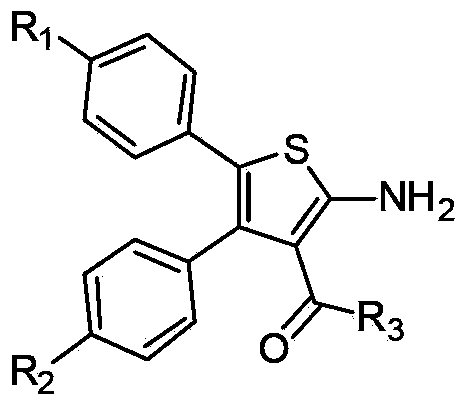
3, 4, 5-tri-substituted aminothiopene chemical compound and preparation and application thereof
A technology of aminothiophene and compounds, which is applied in the field of synthesis of organic compounds, can solve the problems of drug resistance, high toxicity and side effects, etc., and achieve the effect of strong in vitro proliferation inhibition, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1, 1,2-bis(4-chlorophenyl)ethanone (compound IIa):
[0032] Prepared according to literature method (Lütjens et al.J.Med.Chem.2003,46,1870-1877). Put 50ml of dry chlorobenzene in the reaction flask, add 15.46g (100mmol) of 4-chlorophenylacetyl chloride, stir well, then add 40g (300mmol) of aluminum trichloride in batches under ice bath. After the addition, the ice bath was removed, and the mixture was stirred overnight at room temperature. The reaction solution was poured onto ice to quench the reaction, diluted with water, extracted with ethyl acetate (300ml×3), washed with saturated sodium chloride solution (300ml×3), and dried over anhydrous sodium sulfate. After filtration, the filtrate was recovered under reduced pressure to obtain a white solid. After adding petroleum ether (100ml), it was suction-filtered, washed with ice petroleum ether (30ml×3) and ice ether (30ml×3), and dried in vacuo. The yield was 71%.
Embodiment 2
[0033] Example 2, (Z,E)-methyl-3,4-bis(4-chlorophenyl)-2-cyano-2-acetic acid methyl ester (compound IIIa):
[0034]200ml of anhydrous THF was placed in the reaction flask, and 20.6g (110mmol) of titanium tetrachloride was slowly added dropwise under ice cooling. A THF solution (100 ml) of 5.0 g (22 mmol) of compound IIa and 4.3 g (44 mmol) of methyl cyanoacetate was slowly added dropwise to the reaction system. After the addition, remove the ice bath, add 0.8ml of pyridine, stir for 1 hour, then add 2.4ml of pyridine, and stir overnight at room temperature. The reaction solution was evaporated to remove the solvent under reduced pressure, diluted with water, extracted with ethyl acetate (300ml×3), washed with saturated sodium chloride solution (300ml×3), and dried over anhydrous sodium sulfate. After filtration, the filtrate was recovered under reduced pressure to obtain an oil, and the crude product was directly subjected to the next reaction without purification.
Embodiment 3
[0035] Example 3, methyl 2-amino-4,5-bis(4-chlorophenyl)thiophene-3carboxylate (Compound IVa):
[0036] Compound IIIa was dissolved in THF (200ml), added sulfur powder 1.4g (44mmol) and diethylamine 4.0ml, stirred at room temperature for 2h. The reaction liquid was evaporated to remove the solvent under reduced pressure, diluted with water, extracted with ethyl acetate (100ml×3), washed with saturated sodium chloride solution (100ml×3), and dried over anhydrous sodium sulfate. After filtration, the filtrate was recovered under reduced pressure, and the crude product was recrystallized from ethanol to obtain 5.3 g of a white solid, with a two-step yield of 65%.
[0037] MS(m / z): 377.95[M+H] + .
[0038] 1 H NMR (500MHz, CDCl 3 )δ7.26(d,J=8.4Hz,2H),7.11(dd,J=6.4,4.6Hz,1H),7.07(dd,J=6.2,4.5Hz,1H),6.91(d,J=8.5 Hz,2H),6.17(br,2H),3.51(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com