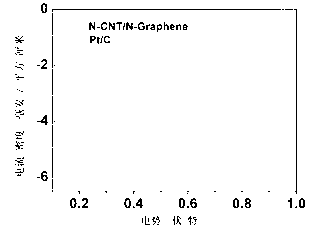
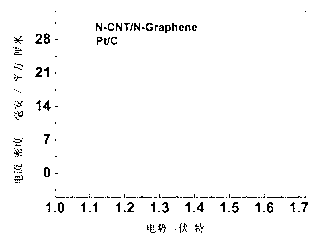
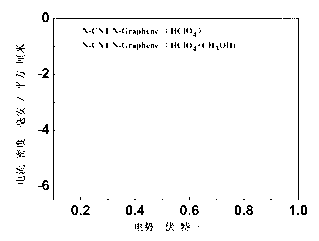
Double-functional catalyst of carbon-based non-noble-metal oxygen electrode and preparation method thereof
A bifunctional catalyst, non-precious metal technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. The problems of poor acceptability and long-term operation stability, low catalytic activity, etc., achieve the effects of easy purchase and preparation, promotion of catalytic performance, and large surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Preparation of Fe-containing non-noble metal catalysts
[0035] Weigh the two substances according to the mass ratio of graphene oxide to vulcan XC-72 carbon black 3:1, and weigh FeSO with the mass ratio of vulcan XC-72 carbon black 2:5 4 .7H 2 O, weigh the nitrogen-containing heterocyclic compound at a mass ratio of 80:1 to vulcan XC-72 carbon black, disperse the above-mentioned substance in solvent water (add a small amount of ethanol to promote dispersion), and vigorously sonicate until it is evenly stirred, and then spin evaporate at 60 °C Dry to obtain a gray-brown powder precursor, put the precursor in a tube furnace under the protection of high-purity Ar inert gas, and pyrolyze it at 900 °C for 1 h to obtain a black Fe- and FeO-containing x Non-precious metal catalysts.
[0036] 2) Test the catalytic performance of Fe-containing non-noble metal catalysts
[0037] An appropriate amount of catalyst powder was uniformly dispersed in Nafion's isopropanol soluti...
Embodiment 2
[0047] 1) Preparation of Fe-containing non-noble metal catalyst with GO (graphene oxide) / C ratio of 4:1
[0048] Weigh the two substances according to the mass ratio of graphene oxide and vulcan XC-72 carbon black 4:1, and weigh FeSO with the mass ratio of vulcan XC-72 carbon black 2:5 4 .7H 2 O, weigh the nitrogen-containing heterocyclic compound at a mass ratio of 80:1 to vulcan XC-72 carbon black, disperse the above-mentioned substance in solvent water (add a small amount of ethanol to promote dispersion), and vigorously sonicate until it is evenly stirred, and then spin evaporate at 60 °C Dry to obtain the gray-brown powdery precursor, put the precursor in a tube furnace under the protection of high-purity Ar inert gas, and pyrolyze it at 900 °C for 1 h to obtain black Fe or FeO x Non-precious metal catalysts.
[0049] 2) Test the catalytic performance of non-precious metal catalysts
[0050] An appropriate amount of catalyst powder was uniformly dispersed in Nafion's i...
Embodiment 3
[0054] 1) Preparation of GO / C 3:2 Fe-containing non-noble metal catalyst
[0055] Weigh the two substances according to the mass ratio of graphene oxide and vulcan XC-72 carbon black 3:2, and weigh FeSO with the mass ratio of vulcan XC-72 carbon black 2:5 4 .7H 2 O, weigh the nitrogen-containing heterocyclic compound at a mass ratio of 80:1 to vulcan XC-72 carbon black, disperse the above-mentioned substance in solvent water (add a small amount of ethanol to promote dispersion), and vigorously sonicate until it is evenly stirred, and then spin evaporate at 60 °C Dry to obtain the gray-brown powdery precursor, put the precursor in a tube furnace under the protection of high-purity Ar inert gas, and pyrolyze it at 900 °C for 1 h to obtain black Fe or FeO x Non-precious metal catalysts.
[0056] 2) Test the catalytic performance of non-precious metal catalysts
[0057] An appropriate amount of catalyst powder was uniformly dispersed in Nafion's isopropanol solution, and the ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com