Binuclear anthracene pyridone sulfonic acid compounds or salts thereof, and preparation method and application thereof
A technique for binuclear anthrapyridone sulfonic acid and compounds, which is applied in the field of structure of binuclear anthrapyridone sulfonic acid compounds, and can solve problems such as unsatisfactory solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
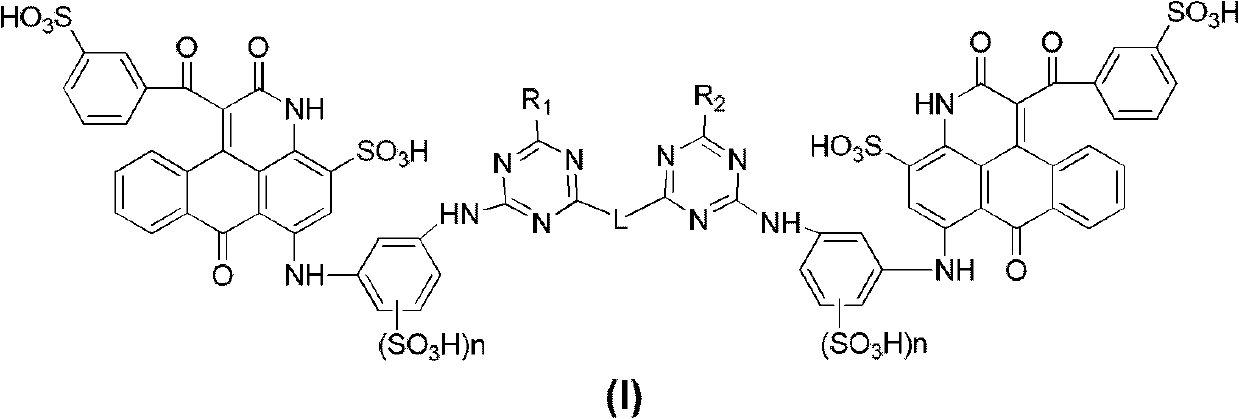
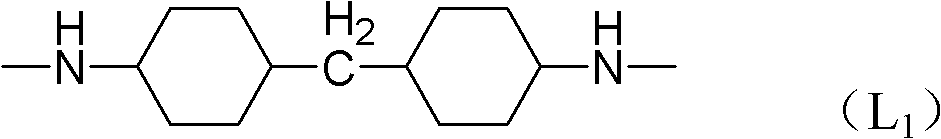
[0067] The preparation method of the dinuclear anthrapyridone sulfonic acid compound of the general formula I, using the mononuclear compound III as the basic raw material, forms a ring in an organic solvent to form the compound IV of the general formula, and then sulfonates and hydrolyzes the compound of the general formula with an amino group The general formula V, then forms the intermediate VI with cyanuric chloride, then reacts with the compound RH to form the general formula VII, and finally connects with the linker H-L-H to form the general formula I. Salt is then converted into salt.
[0068] In the ring-forming reaction of formula III, the reaction condition of the reaction of blue anthraquinone sulfonic acid compound (general formula III) and benzoyl acetate to form compound IV is: 1. compound III and benzoyl acetate (such as formosyl acetate) ester, ethyl ester, propyl ester, isopropyl ester) in an organic solvent at a molar ratio of 1:2-5 at 175-180°C.
[0069] Th...
Embodiment 1
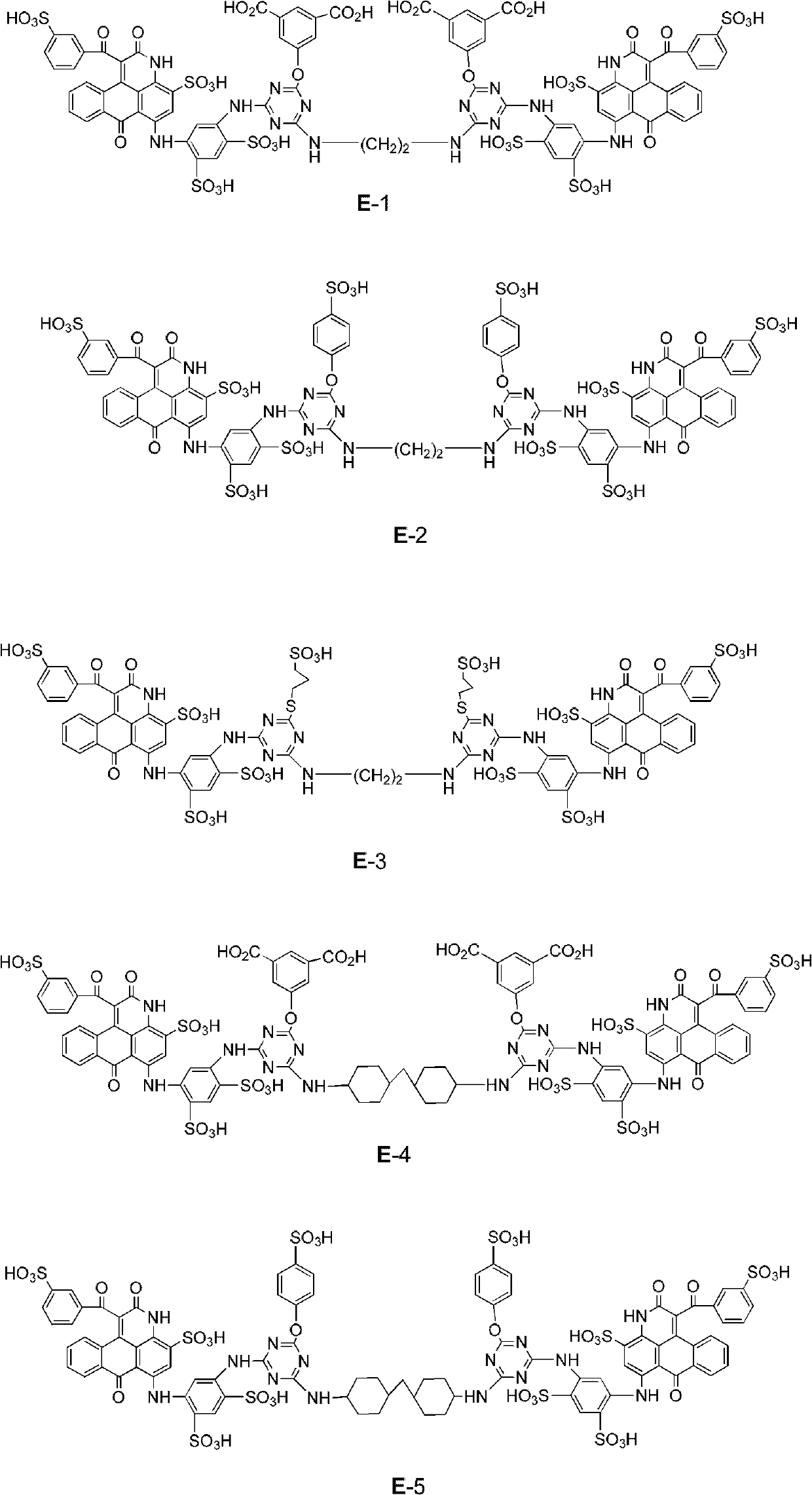
[0104] Example 1. Preparation of Compound E-1
[0105] (1) Add 60 parts of dimethyl sulfoxide to 210 parts of o-dichlorobenzene, and then add 142.2 parts of C.I. acid blue 324 (sodium salt), 3.6 parts of sodium carbonate, and 135.0 parts of ethyl benzoyl acetate under stirring and heat up. React at a temperature of 175 to 180°C for 6 hours. The ethanol and water generated in the reaction are evaporated out of the reaction system through azeotropic distillation, and the color gradually changes from blue to purple. The liquid chromatograph detects that the reaction is complete (it takes about 6 Hour). After cooling, add 300 parts of isopropanol at 30°C and stir for 30 minutes, filter and separate the solid, and wash the resulting filter cake with 400 parts of isopropanol, dry to obtain 155 parts of lavender crystal E1-1 sulfonic acid, sodium salt). Maximum absorption in water at 535 and 560nm, mass spectrum (EI-MS) m / z (-): 578.1 ([ M -H] -1 ). Intermediate dye E1-1 (with ...
Embodiment 2
[0115] Example 2. Preparation of compound E-2
[0116] Compound E1-C1 was prepared according to steps (1)-(3) in Example 1 2
[0117] (4) Containing the above-mentioned E1-C1 2 To the reaction solution, ice was added to adjust the temperature to 5°C. 25% sodium hydroxide aqueous solution was added dropwise to adjust the pH to 5-6. In addition, 13.4 parts of sodium p-hydroxybenzenesulfonate and 25% aqueous sodium hydroxide solution were added to 40 parts of water to adjust the pH to 9 to form an aqueous solution. The aqueous solution of sodium p-hydroxybenzenesulfonate was added dropwise to the above reaction solution at 5° C. over 30 minutes. Continuously add ice and aqueous sodium hydroxide solution, maintain the pH value at 9.0±0.3, raise the temperature to 27-30°C, react at this temperature and pH value for 1 hour, and then react at 40-45°C for 1 hour. After completion of the reaction, water was added to adjust the liquid volume to about 350 parts, and then the insolub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com