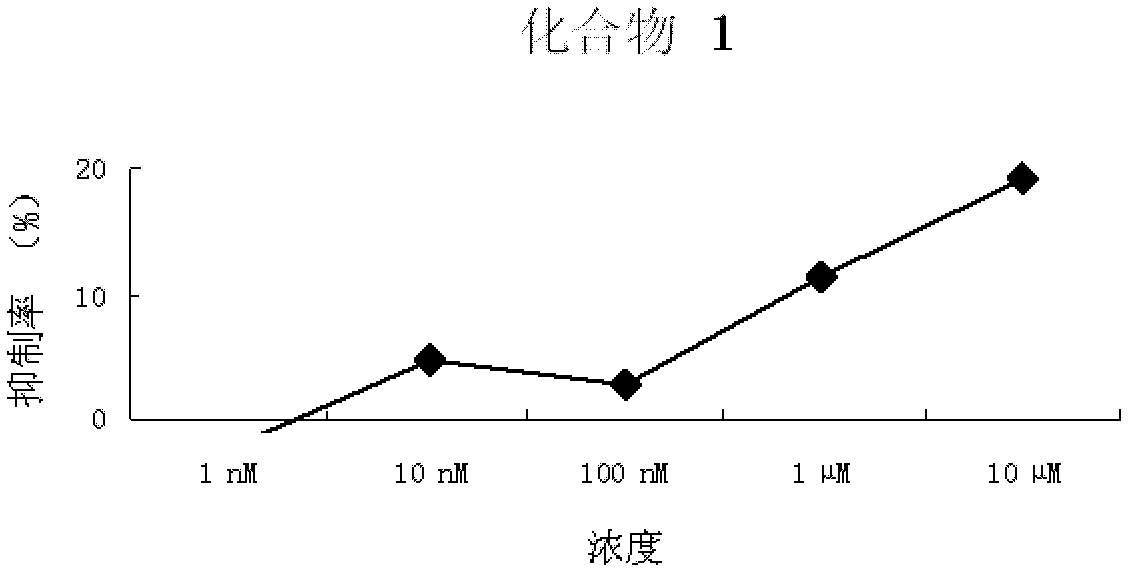
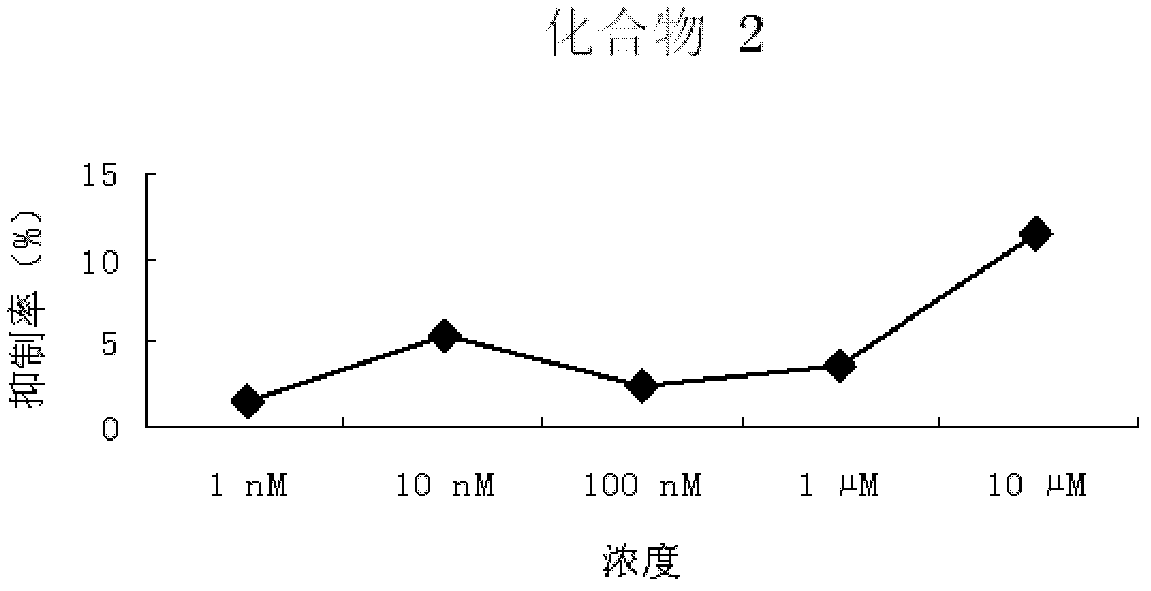
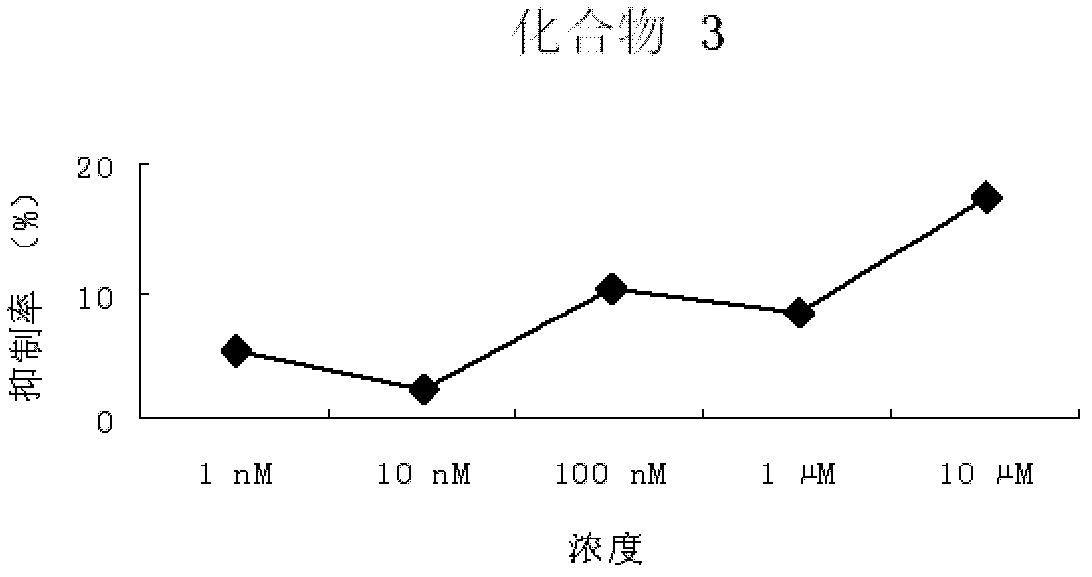
Pyrimidopyrimidine compound, nucleoside analog derivative thereof, preparation method thereof and use thereof
A compound and pyrimidine technology, applied in the field of pyrimidopyrimidine compounds and their nucleoside analog derivatives, can solve the problems of structural gap and low efficiency, and achieve the effect of inhibiting virus replication, inhibiting hepatitis virus, and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of 4,7-diamino-5-methoxypyrimido[4,5-d]pyrimidin-2(1H)-one
[0041] The compound structural formula is as follows:
[0042]
[0043] In an ice bath, add 70 mL of N,N-dimethylformamide dropwise to 300 mL of phosphorus oxychloride, stir slowly for 20 minutes and then put it at room temperature. Add 50 g of commercially available 2-amino-4,6-dihydroxy Pyrimidine, after the reaction is fully stirred, raise the reaction to 110 degrees, remove excess phosphorus oxychloride after two hours of reaction, then slowly pour it into the ice-water mixture (about 8000mL), and then increase the temperature to 50 degrees to obtain The yellow precipitate, after drying, reacts with hydroxylamine hydrochloride in glacial acetic acid to obtain a white precipitate, which is refluxed with selenium dioxide and pyridine in toluene, and the solvent is recovered after the reaction to obtain a light red product, which is then mixed with o-benzene Diformyl chloride was react...
Embodiment 2
[0045] Example 2 Preparation of 4,7-diaminopyrimido[4,5-d]pyrimidine-2,5(1H,6H)-dione
[0046] The compound structural formula is as follows:
[0047]
[0048] 4,7-Diamino-5-methoxypyrimido[4,5-d]pyrimidin-2(1H)-one (200mg, 0.96mmol) was added to 20mL CH 3 In CN, 200 mg sodium iodide and 200 μL trimethylchlorosilane were added to the above mixed solution, stirred overnight at room temperature to obtain a white precipitate 4,7-diaminopyrimido[4,5-d]pyrimidine-2,5(1H , 6H)-diketone. 1H NMR (400MHz, [D6]DMSO): δ7.67-9.08 (m, 4H, 2-NH2), 12.16 (s, 2H, -NH), MS (ESI): calcd for: [C6H6N6O2+H]+ : 195.0631, found: 195.0625.
Embodiment 3
[0049] Example 3 Preparation of 4,7-diamino-1-(β-D-ribofuranose)pyrimido[4,5-d]pyrimidine-2,5(1H,6H)-dione
[0050] The compound structural formula is as follows:
[0051]
[0052] 4,7-Diamino-5-methoxypyrimido[4,5-d]pyrimidin-2(1H)-one (1 g, 4.8 mmol) was added to 100 mL of hexamethyldisilazane, trimethyl chloride Silane (1 ml) was used as a catalyst, and after the reaction was completed, it was glycosylated with 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose in acetonitrile to obtain the nucleus Glycoside, and then remove the protecting group to obtain 4,7-diamino-1-(β-D-ribofuranose)pyrimido[4,5-d]pyrimidine-2,5(1H,6H)-dione. UV (MeOH): 228 (2359), 283 (1216). δH (400MHz; d6-DMSO): 3.41-3.45 (1H, m, OH), 3.59-3.65 (2H, t, 5'CH2), 4.19 ( 1H, s, OH), 4.54-4.56 (1H, d, J=8.0Hz, 2'H), 4.68 (1H, s, 3'H), 4.80-4.81 (1H, bd, J=2.0Hz, OH ), 5.02(1H, s, 4'H), 6.47(1H, s, 1'H), 7.57-8.25(4H, m, NH2×2), 11.72(1H, br, NH).HRMS(ESI- )m / z: Calc.for C11H14N6O6: 325.0896[M-H]-.Foun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com