Ferric ion stabilizing capability measurement method of acidification ferric ion stabilizer
A technology of iron ion stabilizer and determination method, which is applied in the direction of color/spectral characteristic measurement, etc., which can solve the problems of large influence of measurement result storage time, large method error, and low measurement result, so as to facilitate experimental operation and end point judgment , high precision, avoid the effect of repeated heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
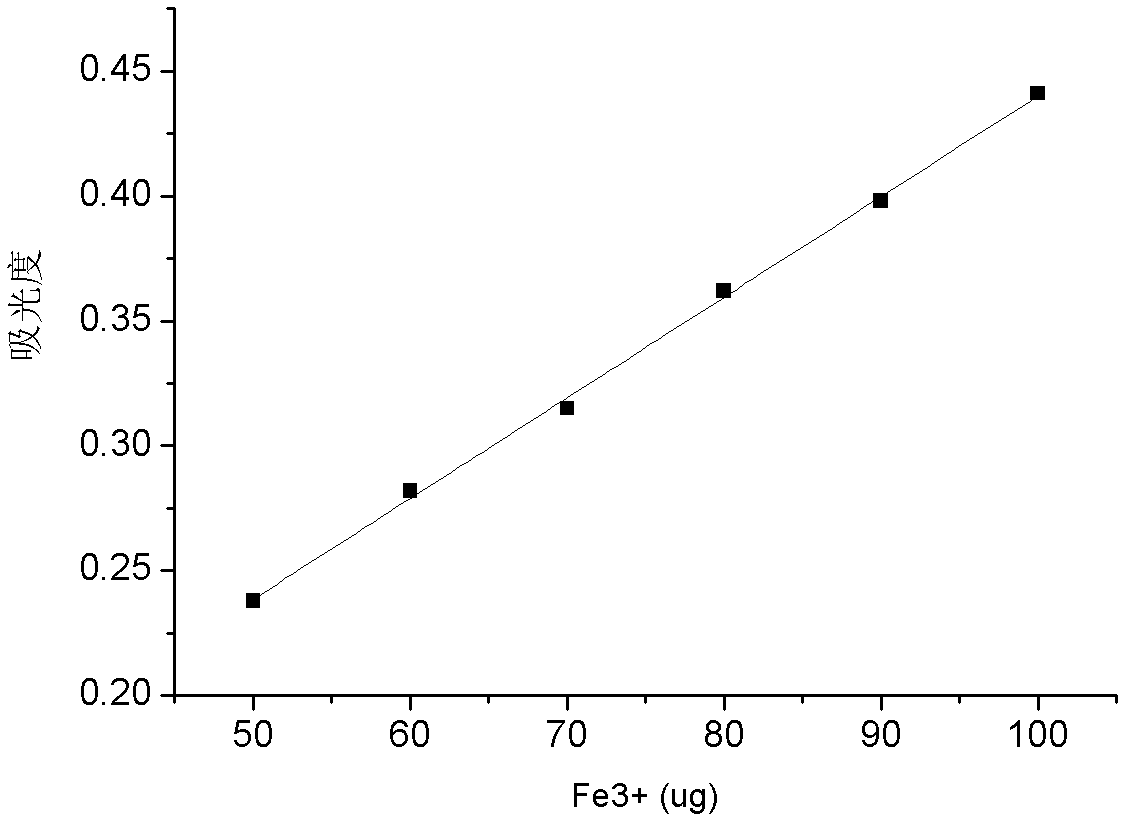
[0034] Reagent preparation
[0035] 5.0mg / mL standard ferric ion solution: Accurately weigh 6.0335g of ferric chloride hexahydrate (FeCl 3 ·6H 2O) in a beaker, after dissolving with distilled water, dissolve it in a 250mL volumetric flask, and shake it up for subsequent use;
[0036] 20% anhydrous sodium carbonate solution: Weigh 20.0g of anhydrous sodium carbonate into a thin-mouth plastic bottle, add 80mL of distilled water, shake well for later use;
[0037] 10% sulfosalicylic acid solution: weigh 10.0g sulfosalicylic acid into a narrow-mouth glass bottle, add 90mL distilled water, shake well for later use;
[0038] Buffer solution with pH=2.2: Pipette 230 mL of 0.2 mol / L hydrochloric acid solution and 250 mL of 0.2 mol / L potassium hydrogen phthalate solution, mix and dilute to 1000 mL with distilled water;
[0039] 0.01mg / mL iron standard solution: Accurately weigh 0.8634g iron ammonium alum [(FeNH 4 (SO 4 ) 2 12H 2 O] Put it in a beaker, add distilled water to diss...
Embodiment 2
[0055] Using this assay method, five different iron ion stabilizers were tested for their ability to stabilize iron ions, and the standard was added for recovery experiments. The results are shown in Table 2. It can be seen from Table 2 that the recovery rate of standard addition is in the range of 100±10%, and the accuracy is better.
[0056] Table 2 Iron ion stabilizer spiked recovery experimental results
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com