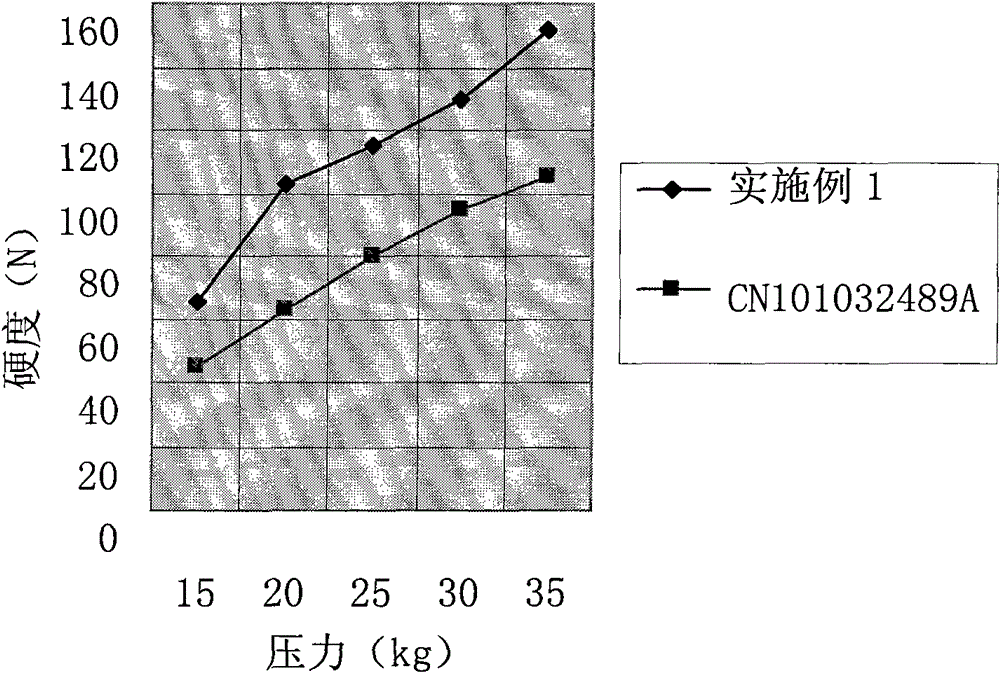
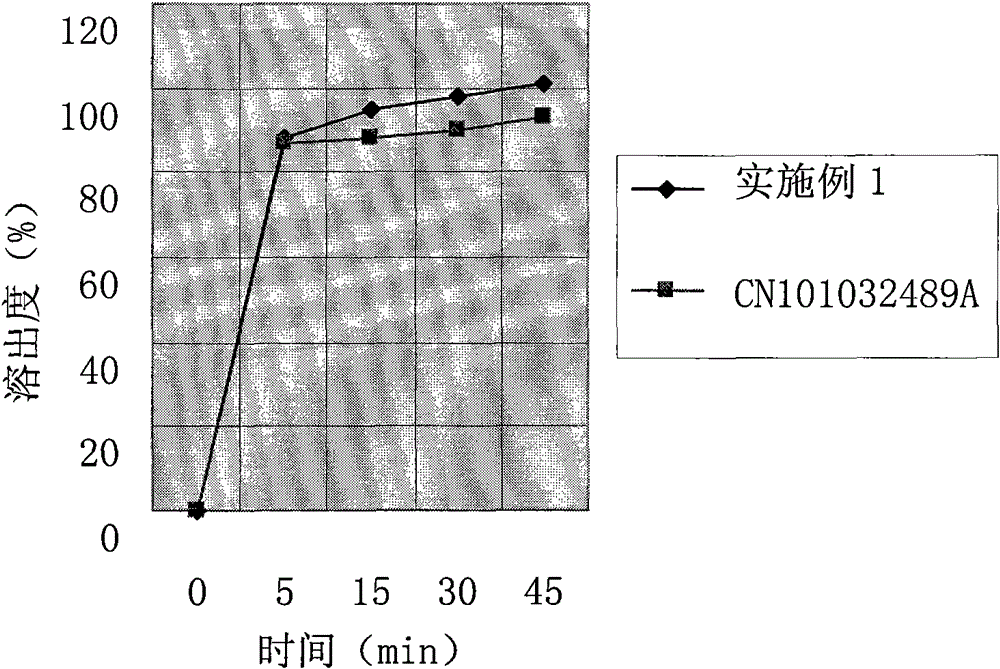
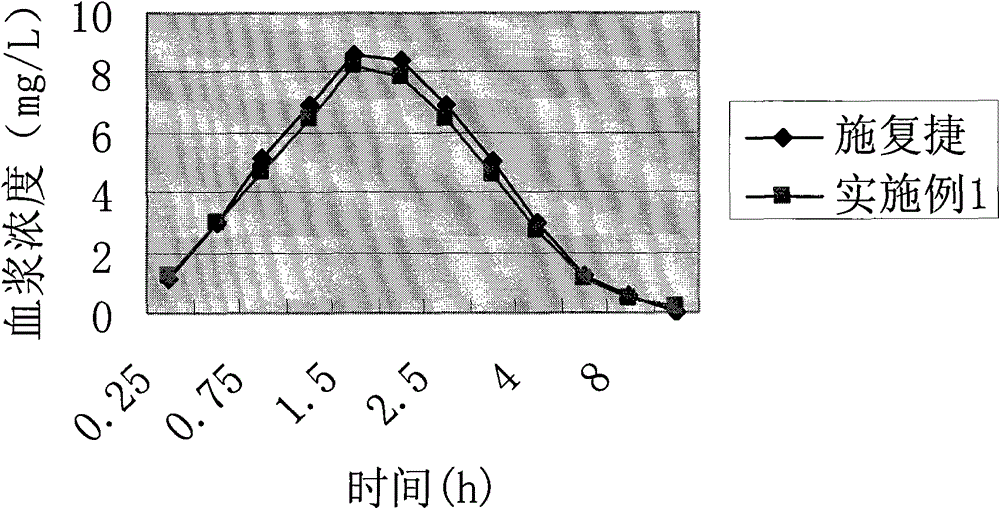
Cefprozil pharmaceutical composition
A technology of cefprozil and composition, applied in the field of medicine, can solve the problem that the hardness of the tablet is difficult to meet the requirements of packaging and transportation, and achieve the effects of excellent dispersion uniformity, release property and excellent hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Dispersible tablets containing 250 mg of cefprozil (calculated as anhydrous substance) as the non-micronized active compound, the cefprozil active compound accounting for about 74% of the total tablet weight:
[0048] Cefprozil (as anhydrous substance) 250mg
[0049] Microcrystalline Cellulose 57mg
[0050] Pregelatinized starch 10mg
[0051] 3% hypromellose 50% alcohol solution 100ml
[0052] Crospovidone 17mg
[0054]Preparation method: Cefprozil, pregelatinized starch, microcrystalline cellulose, 40% crospovidone (6.8mg), granulate with the hypromellose ethanol solution, dry, then add the remaining crospovidone Lupovidone, Magnesium Stearate, Blended, Tabletted.
Embodiment 2
[0056] Dispersible tablets containing 250 mg of cefprozil (calculated as anhydrous substance) as the non-micronized active compound, the cefprozil active compound accounting for about 68% of the total tablet weight:
[0057] Cefprozil (as anhydrous substance) 250mg
[0058] Microcrystalline Cellulose 91.2mg
[0059] Pregelatinized Starch 4mg
[0060] 3% hypromellose 50% alcohol solution 100ml
[0061] Crospovidone 11mg
[0062] Magnesium Stearate 1.8mg
[0063] Preparation method: add 5.5 mg of crospovidone first (accounting for 50% of the total weight of crospovidone), and the others are the same as in Example 1.
Embodiment 3
[0065] Dispersible tablets containing 250 mg of cefprozil (calculated as anhydrous substance) as non-micronized active compound, the cefprozil active compound accounting for about 80% of the total tablet weight:
[0066] Cefprozil (as anhydrous substance) 250mg
[0067] Microcrystalline Cellulose 32mg
[0068] Pregelatinized Starch 4mg
[0069] 2% hypromellose 50% alcohol solution 80ml
[0070] Crospovidone 12mg
[0071] Magnesium Stearate 4mg
[0072] Preparation method: add 4.2 mg of crospovidone first (accounting for 35% of the total weight of crospovidone), and the others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com