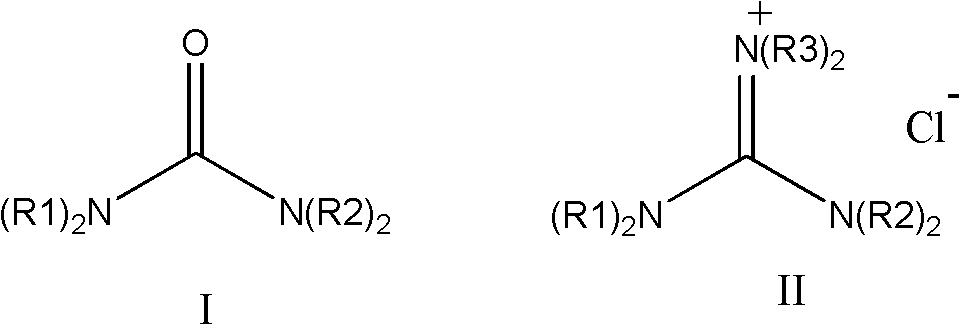
Method for preparing and purifying hexaalkylguanidine chloride
A purification method and chloride technology are applied in the field of preparation and purification of phase transfer catalyst hexaalkylguanidine chloride, which can solve the problems of difficult high-temperature polymerization of liquids, restricting the application of hexaalkylguanidine salts, affecting polymer quality and the like , to achieve the effect of easy transportation and storage, meeting the requirements of high temperature polymerization, and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
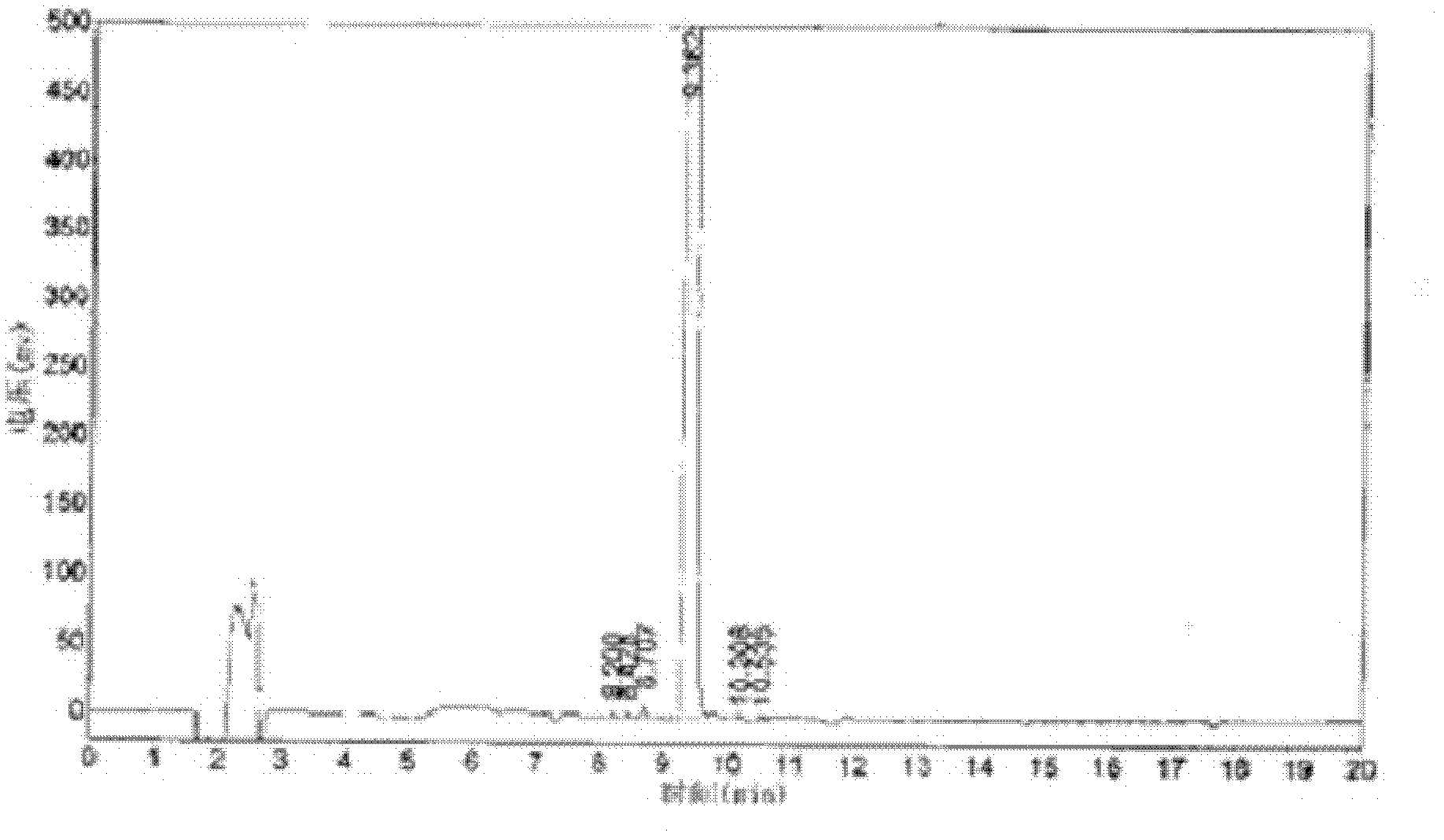
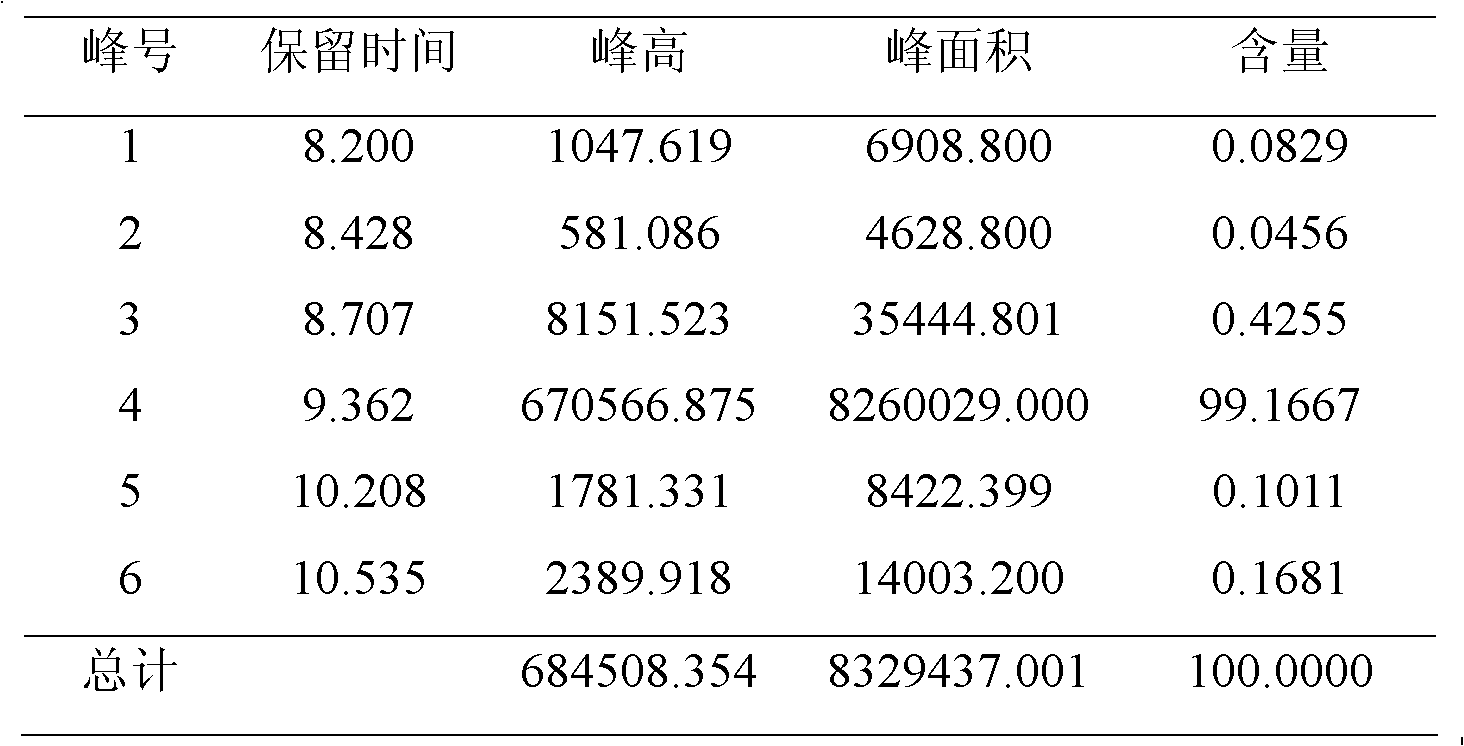
[0041] Under nitrogen protection, add tetraethylurea (43g, 0.25mol) in a 500ml four-necked flask equipped with stirring paddle, thermometer, condenser tube and nitrogen inlet tube, dry toluene (25ml), stir to make it dissolve, and Molecular sieves type HZSM-5 (1.25 g) were added. Then a solution of triphosgene (29.7 g, 0.10 mol) in toluene (125 ml) was added dropwise. After the dropwise addition, the temperature was raised to 60° C., and the stirring reaction was continued for 2 h. After the reaction was completed, the temperature was lowered to 0°C. Under nitrogen protection, diethylamine (43.8g, 0.6mol) was added dropwise, the reaction temperature was controlled at 20°C to 26°C, monitored by HPLC, after the reaction was finished, the pH value was adjusted to about 7.5 with 50wt% sodium hydroxide aqueous solution (100ml), Separation, water phase with CH 2 Cl 2 (50ml×5) extraction, combined CH 2 Cl 2 phase, dried over anhydrous magnesium sulfate, and the solvent was disti...
Embodiment 2
[0045] Under nitrogen protection, add tetramethylurea (75.4g, 0.65mol) to a 1L four-necked flask equipped with stirring paddle, thermometer, condenser tube and nitrogen inlet tube, dry toluene (110ml), stir to make it dissolve, And add HZSM-5 type molecular sieve (3.25g). Then a solution of triphosgene (80 g, 0.27 mol) in toluene (300 ml) was added dropwise. After the dropwise addition, the temperature was raised to 60° C., and the stirring reaction was continued for 2.7 h. After the reaction was completed, the temperature was lowered to 0°C. Under nitrogen protection, dropwise add dimethylamine (73g, 1.62mol), control reaction temperature at 20 ℃~26 ℃, HPLC monitors, after reaction finishes, adjust pH value to about 7.5 with 50wt% sodium hydroxide aqueous solution (280ml), divide Liquid, water phase with CH 2 Cl 2 (135ml×5) extraction, combined CH 2 Cl 2 phase, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a ye...
Embodiment 3
[0047] Under nitrogen protection, add tetraethylurea (250g, 1.45mol) to a 5L four-necked flask equipped with a stirring paddle, a thermometer, a condenser tube and a nitrogen inlet tube, dry toluene (900ml), stir to dissolve it, add Zeolite H Beta (7.25 g). Then a solution of triphosgene (180 g, 0.61 mol) in toluene (750 ml) was added dropwise. After the dropwise addition, the temperature was raised to 60° C., and the stirring reaction was continued for 3 h. After the reaction was completed, the temperature was lowered to 0°C. Under nitrogen protection, dropwise add diethylamine (270g, 3.70mol), control reaction temperature at 20 ℃~26 ℃, HPLC monitors, after reaction finishes, adjust pH value to about 7.5 with 50wt% sodium hydroxide aqueous solution (660ml), divide Liquid, water phase with CH 2 Cl 2 (300ml×5) extraction, combined CH 2 Cl 2 phase, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a yellow oil. It wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com