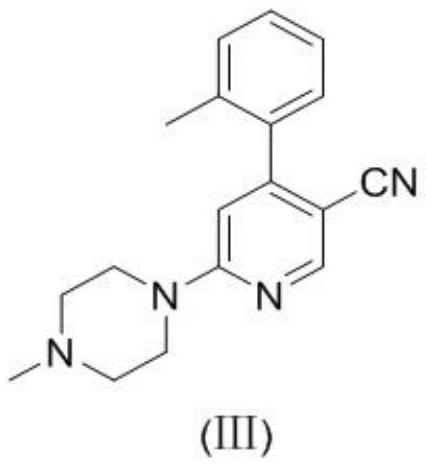
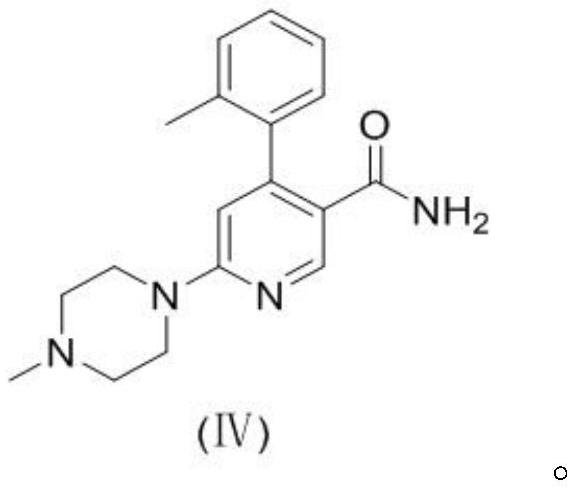
Synthesis method of 6-(4-methylpiperazine-1-yl)-4-o-tolyl nicotinamide
A technology of phenylnicotinamide and a synthesis method, applied in directions such as organic chemistry, can solve problems such as complicated steps and increased cost, and achieve the effects of fewer steps in a reaction process, mild reaction conditions, and safety and environmental protection in the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
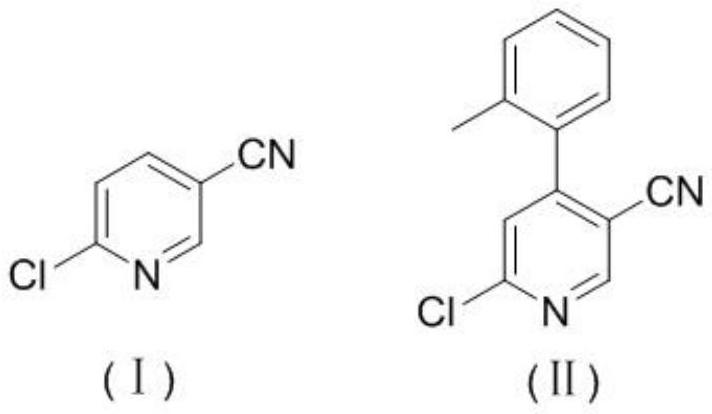
[0037] Step (1) Synthesis of 4-orthodontic-6-Nitrile (II)
[0038] 6-chloroboxia (1.00 g, 7.2 mmol), anhydrous THF (5 mL) was added to 25 ml of three-necked flask, and stirred under nitrogen protection to dissolve, and the ice salt bath was at 0 ° C. Boron tedifer (1.13 g, 7.9 mmol) was added using a 5 ml syringe to maintain this temperature for 20 min. LiCl (0.42 g, 10.0 mmol) was added and stirring was continued for 5 min. Then, the liquid nitrogen is reduced to the temperature to reach -30 ° C. Under nitrogen protection was added to the syringe (8.6 mL, 1.0 M F, 8.6 mmol), and the internal temperature was -30 ° C. The temperature was stirred for 1 h. TLC monitoring shows that the raw material is completely transformed, and it is restored to room temperature. Dichloride (3.27 g, 14.4 mmol) was added, and stirring was continued for 2 h at the same temperature. After the TLC monitoring was complete, the reaction was added to the saturated ammonia (8 ml). Extraction was carried ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com