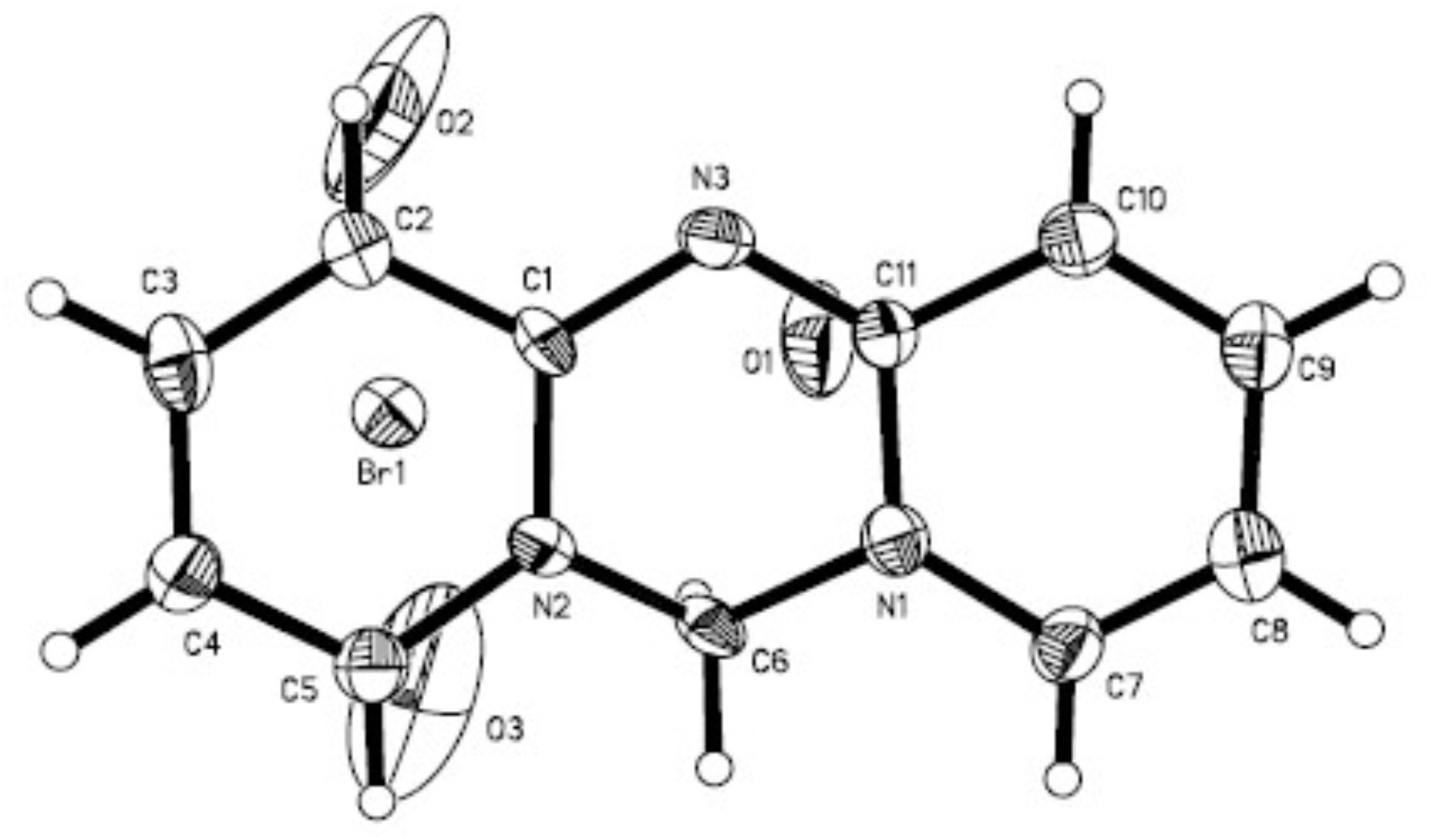
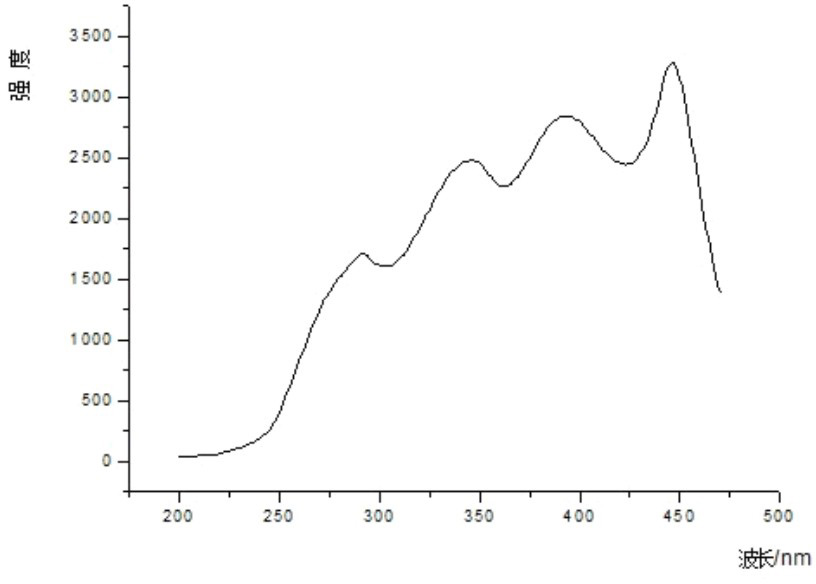
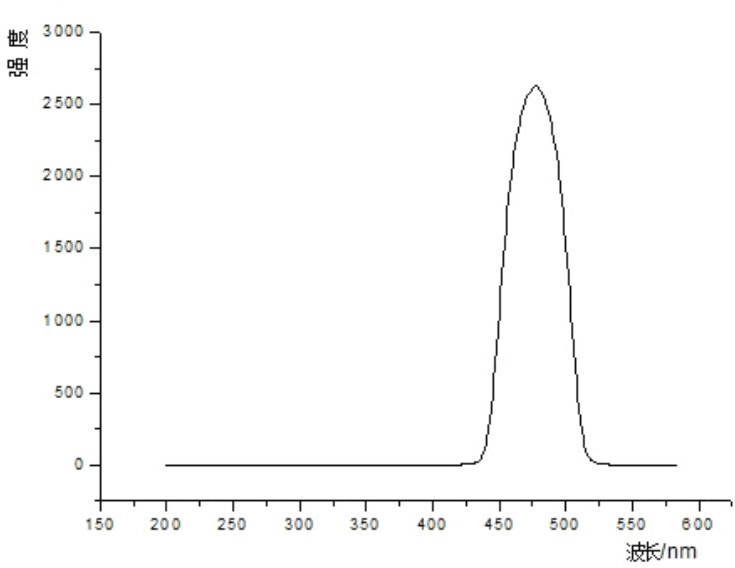
Method for synthesizing hydrated dipyridino hexahydro triazine bromide salt
A technology for hydrating bipyridine bromide and hexahydrotriazine, which is applied in the synthesis of pyridine N-heterocycles and dibromomethane, and in the field of synthesizing hydrated bipyridino-hexahydrotriazine bromide, which can solve the problem that the yield is only 25%. Methyl iodide is expensive, highly toxic and other issues, to achieve the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1.0002g of 2-aminopyridine into a 100mL flask, add 15.0mL of dibromomethane, connect the reflux device, and react in an oil bath at 110°C for 3 hours. After the reaction, cool the flask to room temperature. The remaining dibromomethane was distilled off under reduced pressure to obtain a bright yellow solid powder, which was dissolved in methanol and recrystallized at room temperature to obtain 1.5238 g of light yellow crystals with a yield of 90.20%.
Embodiment 2
[0023] Add 1.5332g of 5-bromo-2-aminopyridine into a 100mL flask, and add 10.0mL of dibromomethane, connect the reflux device, and react in an oil bath at 110°C for 3 hours. After the reaction, cool the flask to room temperature. The remaining dibromomethane was distilled off under reduced pressure, and 1.8390 g of light yellow solid powder was obtained after drying, with a yield of 87.25%.
Embodiment 3
[0025] 2. Add 0128g of 3,5-dibromo-2-aminopyridine into a 100mL flask, add 5.0mL of dibromomethane, connect the reflux device, and react in an oil bath at 120°C for 3 hours. After the reaction, cool the flask to room temperature. The remaining dibromomethane was distilled off under reduced pressure, and 2.0147 g of brown solid powder was obtained after drying, with a yield of 79.61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com