Preparation technology of desogestrel and its new intermediate compound
A technology for desogestrel and steroids, applied in the directions of steroids, organic chemistry, etc., can solve problems such as multiple environmental pollutants, and achieve the effects of less pollutant discharge, high yield, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
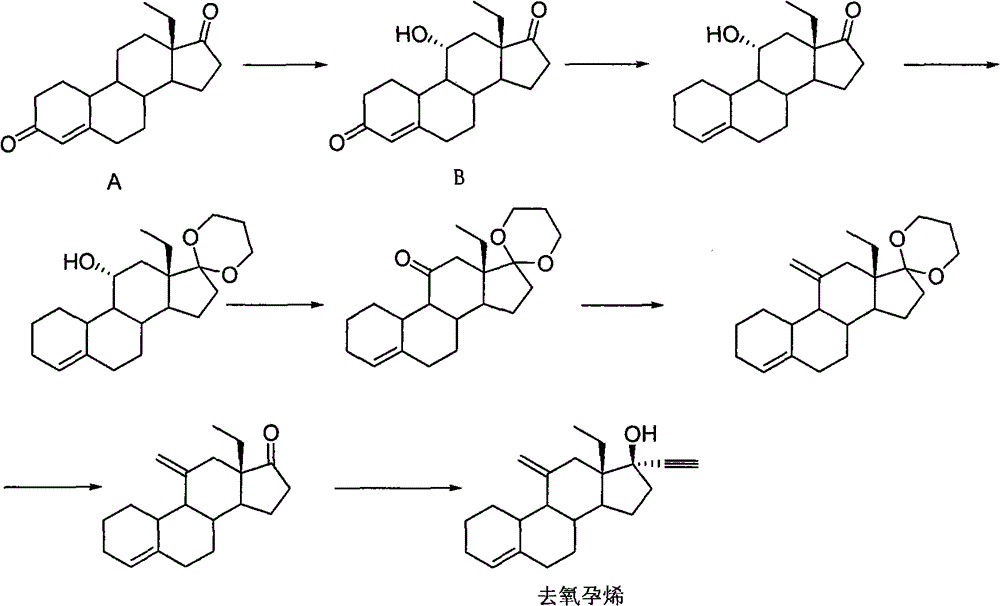
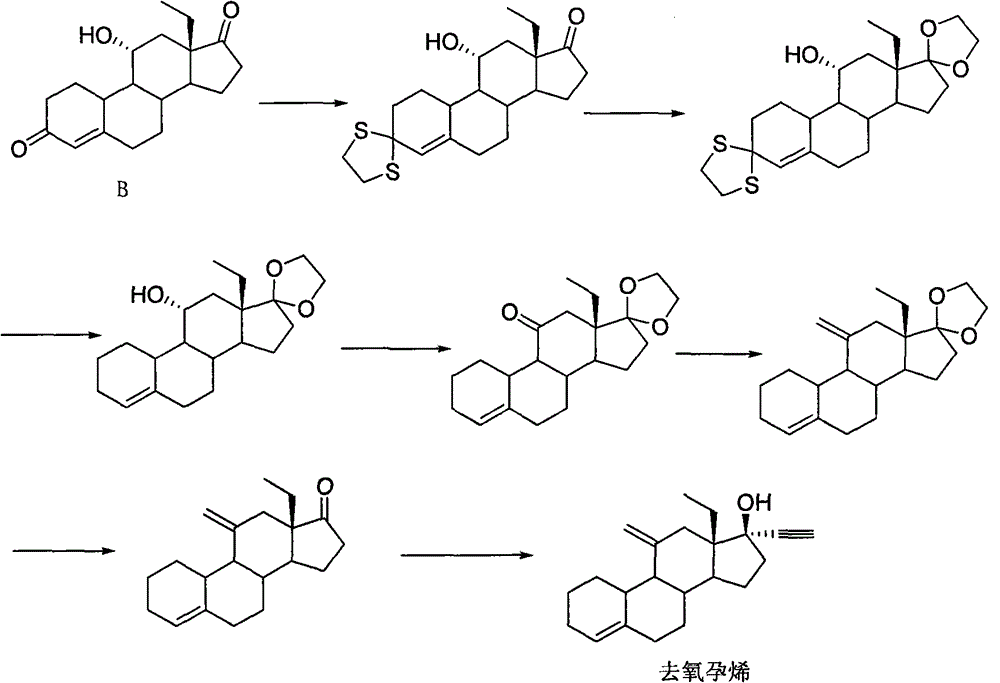
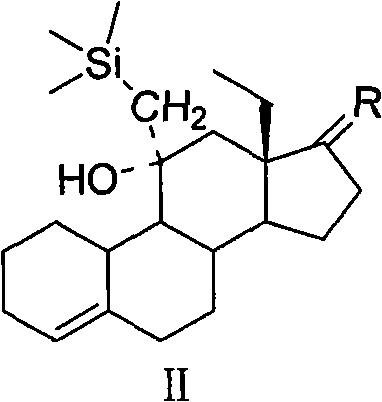
[0038] Example 1: 11β-Hydroxy-11α-(trimethylsilylmethyl)-17,17-ethylenedioxy-18-methyl-estr-4ene
[0039]
[0040] In the there-necked flask, add compound Ia (20.0g, 60.6mmol), n-hexane (470mL), pass nitrogen protection, under stirring at room temperature, slowly dissolve the n-hexane solution of trimethylsilylmethyllithium (130mL, 0.546mol / L ) into the above system, heated in an oil bath for 1.5 hours, lowered the temperature, added 200 mL of water to terminate the reaction, separated the liquids, extracted the water phase twice with 200 mL of n-hexane, combined the n-hexane phase, washed twice with 200 mL of saturated brine, Magnesium sulfate (20.0 g) was dehydrated, filtered, the filtrate was concentrated to dryness, and recrystallized from methanol to obtain compound IIa (22.0 g, 52.6 mmol) as a white solid with a yield of 86.8%. MS(m / z): 418[M] + ; 1 H-NMR, δ0.10(9H, s, -S i (CH 3 ) 3 ), δ1.08 (3H, t, 18-CH 3 ), δ5.41(1H, d, 4-H); 13 C-NMR, 120.2 (C 4 ), 141.7 ...
Embodiment 2
[0041] Example 2: 11β-Hydroxy-11α-(trimethylsilylmethyl)-17,17-(1,3-propanedioxy)-18-methyl-estr-4ene
[0042]
[0043] In a three-neck flask, add cyclohexane (600 mL), compound Ib (20.0 g, 58.1 mmol), trimethylsilylmethylmagnesium chloride tetrahydrofuran solution (140 mL, 1.3 mol / L), and heat the reaction in an oil bath for 2 hours. Lower the temperature, add 200 mL of water to terminate the reaction, separate the liquids, extract the water phase twice with 200 mL of cyclohexane, combine the cyclohexane phase, wash twice with 200 mL of saturated brine, dehydrate with anhydrous magnesium sulfate (20.0 g), filter, and the filtrate Concentrate to dryness and recrystallize from ethanol to obtain white solid IIb (22.5 g, 52.0 mmol), yield 89.5%.
Embodiment 3
[0044] Example 3: 11-methylene-18-methyl-estr-4-en-17-one
[0045]
[0046] In a three-necked flask, compound IIa (20.0 g, 47.8 mmol) and methanol (400 mL) were added, under stirring at room temperature, 2.4 mL of concentrated hydrochloric acid was added, and the reaction was heated in a water bath for 1.5 h. Cool down, add 2.4 g of sodium bicarbonate to neutralize the reaction solution, add the reaction solution to 800 mL of water, filter, drain the filter cake, and recrystallize from methanol to obtain white solid compound 3 (13.0 g, 45.8 mmol), with a yield of 95.8%. MS(m / z): 284[M] + ; 1 H-NMR, δ0.76 (3H, t, 18-CH 3 ), δ4.83 (1H, s, =CH 2 ), δ4.92 (1H, s, =CH 2 ), δ5.48 (1H, d, 4-H). 13 C-NMR, 218 (C 17 )146(C 11 ), 139 (C 5 ), 121(C 4 ), 110 (=CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com