Tablet
A tablet and methyl technology, applied in the field of tablets, can solve problems such as increasing tablet size and decreasing patient compliance, achieve excellent productivity, prevent tablet surface damage, prevent tablet cracking and tablet compression problems Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0175] In particular, preferred embodiments of the tablet of the present invention include:
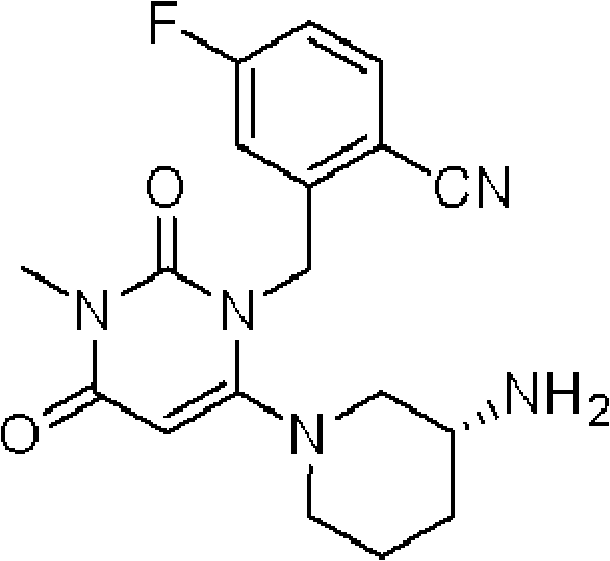
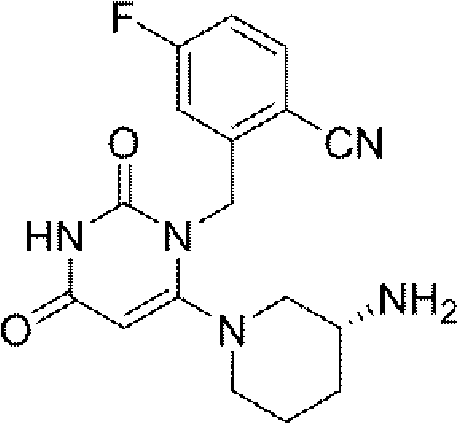
[0176] "Tablets each containing 50 mg of compound (A) (free form)";
[0177] "Tablets each containing 100 mg of Compound (A) (free form)"; and
[0178] "Tablets each containing 200 mg of compound (A) (free form)".
[0179] Compound (A) or a salt thereof may be used in combination with one or more other drugs (hereinafter sometimes referred to as "concomitant drug").
[0180] As a specific example, compound (A) or a salt thereof can be used in combination with one or more drugs (concomitant drugs) selected from the following: therapeutic agents for diabetes, therapeutic agents for diabetic complications, therapeutic agents for hyperlipidemia, anti-hyperlipidemia Blood pressure drugs, anti-obesity drugs, diuretics, anticoagulants, etc.
[0181]Examples of the diabetes therapeutic agent include insulin preparations (for example, animal insulin preparations extracted from bovine or por...
Embodiment 1
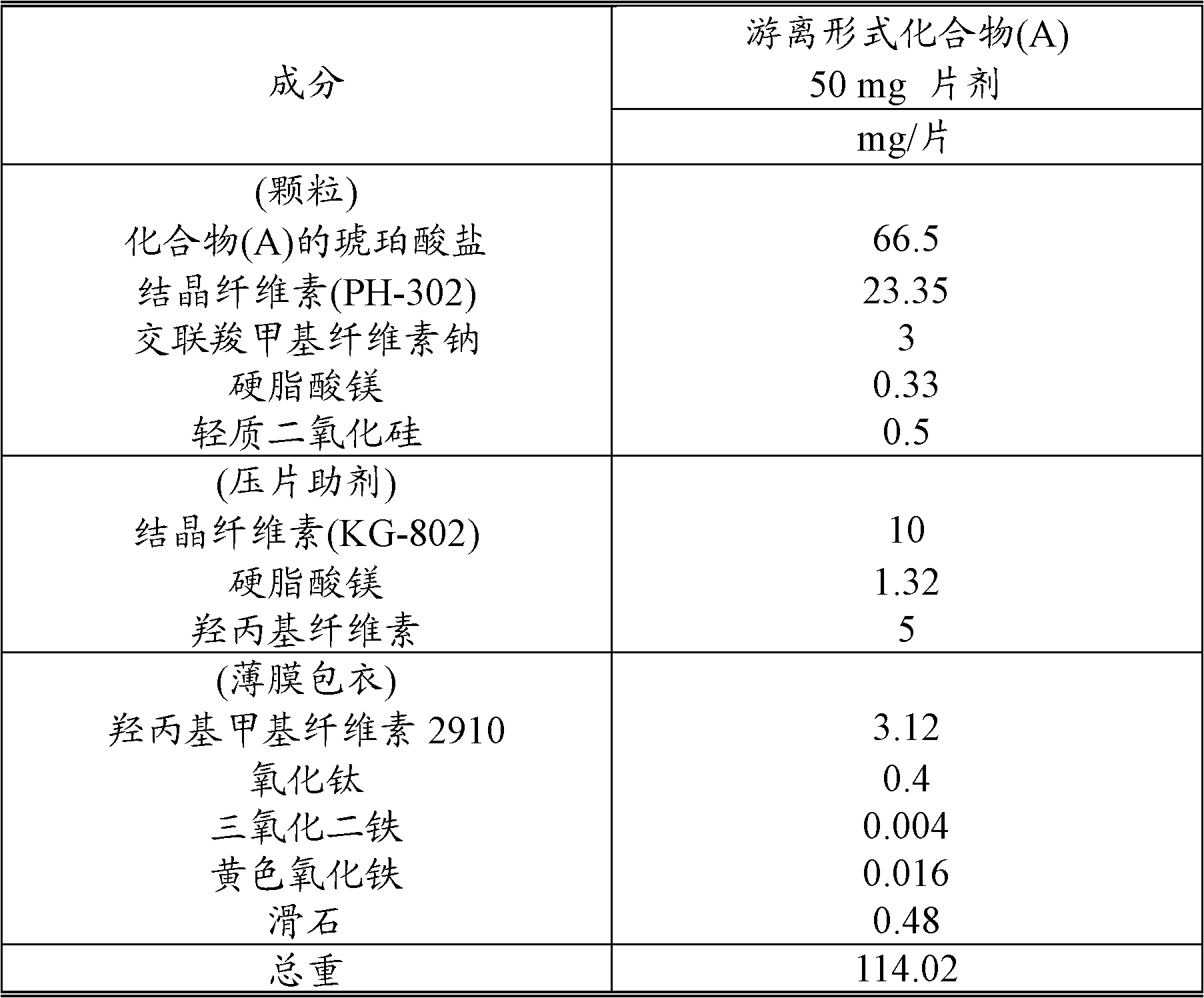
[0196] According to the prescription of Table 1, compound (A) succinate (monosuccinate), crystalline cellulose (PH-302), croscarmellose sodium, magnesium stearate and light silica Mix uniformly in a mixer (vertical granulator 50L, Powrex Corporation) and granulate with a roller compactor (Alexander). The resulting granulated product was granulated by a granulator (power mill P-3, Showa Kako Corporation) to obtain a granulated powder. Crystalline cellulose (KG-802), hydroxypropyl cellulose (HPC-L 100M; Nippon Soda Co., Ltd.) and magnesium stearate were added to the granulated powder, and mixed in a mixer (tumbler 60L , Showa Kako Corporation) was mixed to obtain granules for tableting. The granules were compressed to a weight of 110 mg in a rotary tableting machine (AQUARIOUS3-A, Kikusui Seisakusho Ltd.) with an 8×4.5 mm punch to obtain plain tablets. Titanium dioxide, ferric oxide, yellow iron oxide, and talc were dispersed in an aqueous solution of hydroxypropyl methylcellu...
Embodiment 2
[0200] According to the prescription of Table 2, compound (A) succinate (monosuccinate), crystalline cellulose (PH-302), croscarmellose sodium, magnesium stearate and light silica Mix uniformly in a mixer (vertical granulator 50L, Powrex Corporation) and granulate with a roller compactor (Alexander). The resulting granulated product was granulated by a granulator (power mill P-3, Showa Kako Corporation) to obtain a granulated powder. Crystalline cellulose (KG-802), hydroxypropyl cellulose (HPC-L 100M; Nippon Soda Co., Ltd.) and magnesium stearate were added to the granulated powder, and mixed in a mixer (tumbler 60L , Showa Kako Corporation) was mixed to obtain granules for tableting. The granules were compressed to a weight of 220 mg in a rotary tableting machine (AQUARIOUS3-A, Kikusui Seisakusho Ltd.) with a punch of 11×6 mm to obtain plain tablets. Titanium dioxide, ferric oxide, yellow iron oxide, and talc were dispersed in an aqueous solution of hydroxypropyl methylcell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com