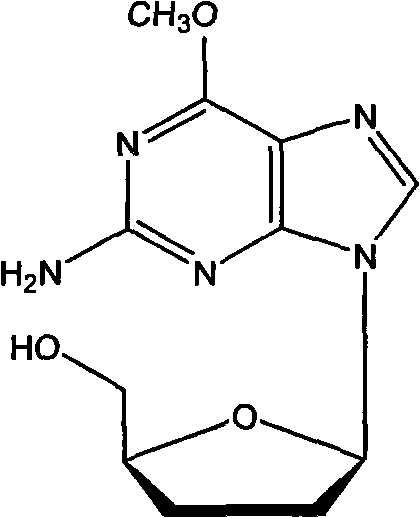
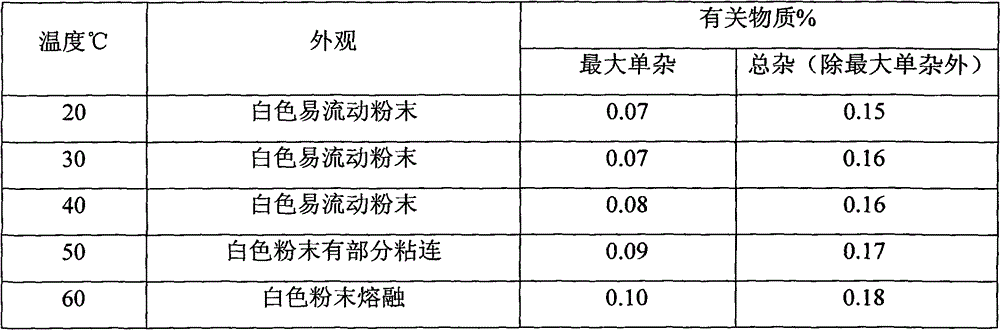
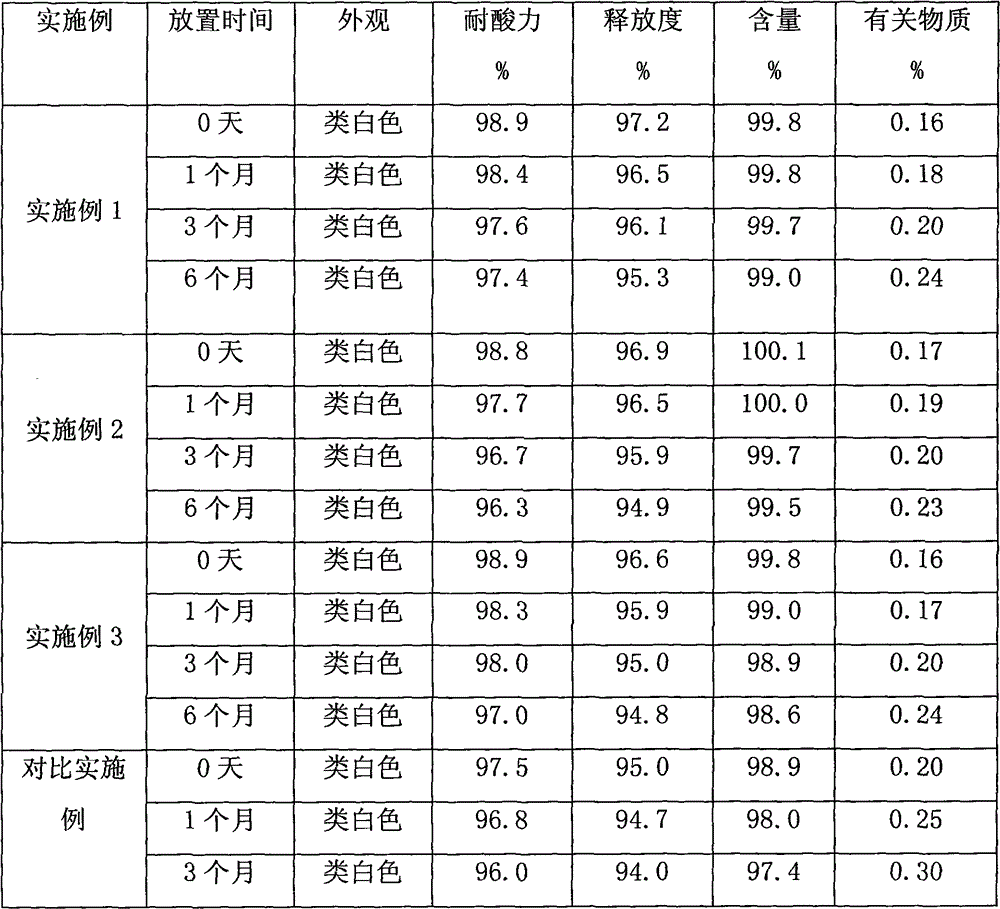
Metacavir enteric-coated pellets and preparation method thereof
A technology of Cavir sausage and pellets, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can solve problems such as temperature instability, poor water solubility of ddG, and difficulty, and achieve Simple operation, avoiding the effect of electrostatic adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A. Drug-containing pellets
[0052] Metacavir 70g
[0053] Microcrystalline Cellulose 105g
[0054] 25% ethanol aqueous solution 140ml
[0055] Preparation Process:
[0056] (1) Metacavir and microcrystalline cellulose are respectively passed through a 100-mesh sieve, mixed uniformly, and soft materials are prepared with 25% ethanol aqueous solution;
[0057] (2) Put the above-mentioned soft material in an extruding spheronizer, and extrude-spheronize-dry to form pellets. The extrusion speed was 15Hz, the spheronization speed was 26Hz (18 minutes), and finally dried at 40°C for 2 hours to obtain 0.6mm drug-containing pellets.
[0058] B. Isolation layer
[0059] Drug-containing pellets 175g
[0060] Hydroxypropyl Methyl Cellulose 14g
[0061] 70% ethanol aqueous solution 105ml
[0062] Preparation Process:
[0063] (1) Disperse the above-mentioned hydroxypropyl methylcellulose in absolute ethanol, then add water to dissolve, and get final product;
[0064] (2)...
Embodiment 2
[0077] A. Drug-containing pellets
[0078] Metacavir 65g
[0079] Lactose 30g
[0080] Microcrystalline cellulose 90g
[0081] 50% ethanol aqueous solution 200ml
[0082] Preparation Process:
[0083] (1) Metacavir, lactose, and microcrystalline cellulose are mixed evenly by the method of equal addition, passed through an 80-mesh sieve, and set aside;
[0084] (2) Use 50% ethanol aqueous solution as adhesive
[0085] (3) Start the centrifugal granulator to prepare drug-containing pellets. The spray speed changes with the granulation time, the first 2min is 100r.min -1 , so that the powder is wetted in a short time to avoid flying dust → adjust the spray speed to 40r.min -1, , continue until the material is in a flocculent flow state → supply powder and control the powder-slurry ratio to make the material flow in a flocculent state → grow into the target pellet core → dry → sieve to obtain drug-containing pellets;
[0086] B. Isolation layer
[0087] Drug-containing pe...
Embodiment 3
[0106] A. Drug-containing pellets
[0107] Metacavir 50g
[0108] Microcrystalline cellulose 50g
[0109] water 85g
[0110] Preparation Process:
[0111] (1) Mix metacavir and microcrystalline cellulose uniformly by equal addition method, and prepare soft material with water as binder;
[0112] (2) Put the above-mentioned soft material in an extruding spheronizer, and extrude-spheronize-dry to form pellets. The extrusion speed was 15Hz, the spheronization speed was 26Hz (18 minutes), and finally dried at 40°C for 2 hours, and the 0.6mm drug-containing pellets were sieved.
[0113] B. Isolate pellets
[0114] Drug-containing pellets 100g
[0115] Hypromellose 8g
[0116] 75% ethanol 80g
[0117] Preparation Process:
[0118] (1) Disperse hydroxypropyl methylcellulose in absolute ethanol first, and then add water to dissolve it;
[0119] (2) Start the fluidized bed, the inlet air temperature is 35°C, the outlet air temperature is 27°C, the material temperature is 30°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com