Tumor targeting magnetic resonance contrast medium modified by chlorotoxin and preparation method and application thereof
A magnetic resonance contrast agent and tumor targeting technology, which is applied in the biological field to achieve the effects of high relaxation rate, reduced dosage and high value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
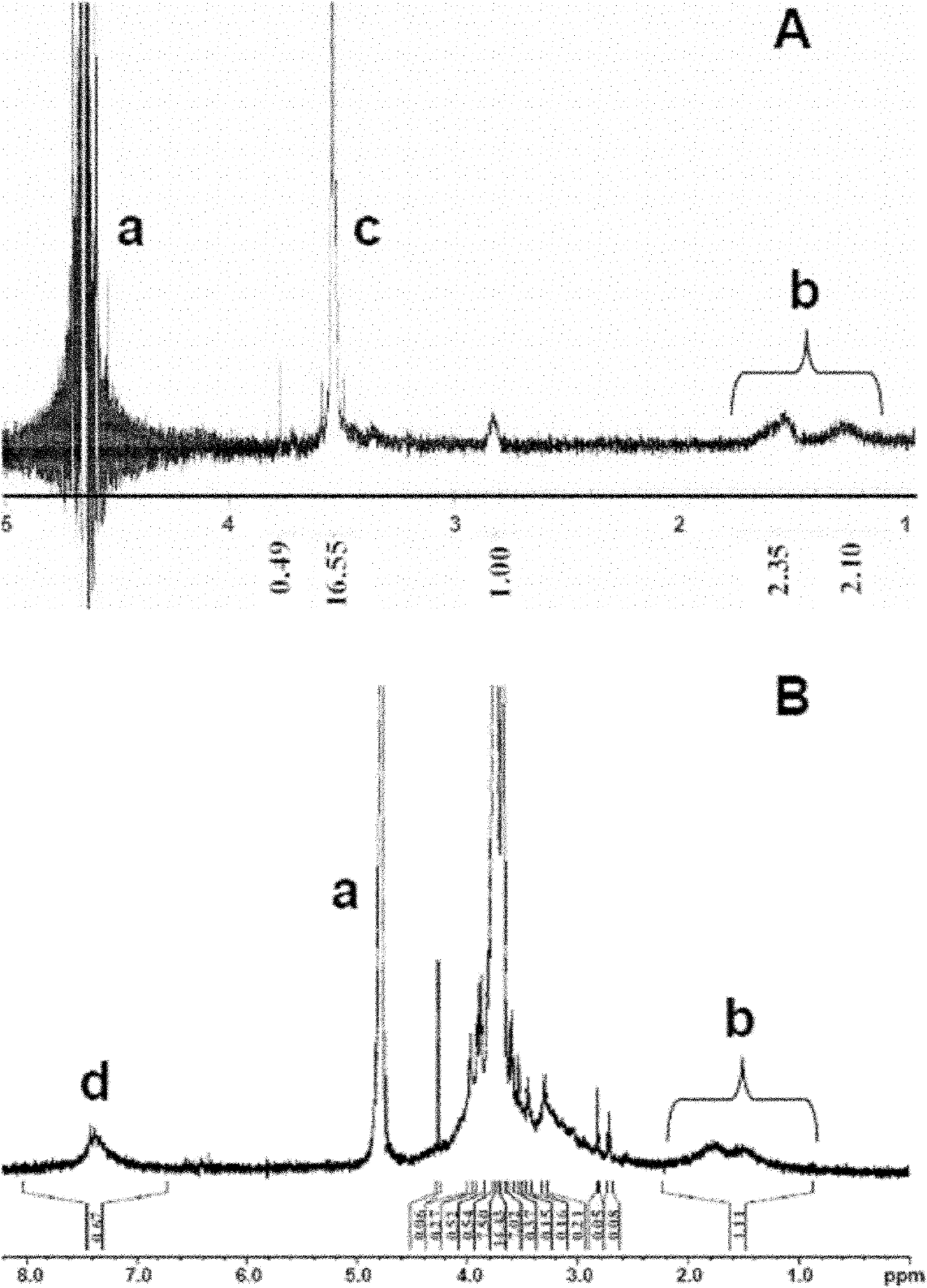
[0075] DGL (second generation) and PEG containing bifunctional groups were dissolved in phosphate buffer saline (PBS for short) at pH 8.0 according to the molecular ratio of 1:2, and stirred at room temperature for 2 h to generate DGL-PEG, which was removed by ultrafiltration. React with PEG and replace buffer with PBS pH 7.0. At the same time, CTX was reacted with Traut's reagent to introduce sulfhydryl groups, mixed with DGL-PEG at a molecular ratio of 1:1, stirred and reacted at room temperature for 24 hours, and unreacted CTX was removed by size exclusion chromatography to obtain DGL-PEG. PEG-CTX.
Embodiment 2
[0077] DGL (second generation) and PEG containing bifunctional groups were dissolved in PBS at pH 8.0 according to the molecular ratio of 1:5, and stirred at room temperature for 2 hours to generate DGL-PEG, and the unreacted PEG was removed by ultrafiltration and the buffer was replaced PBS pH 7.0. At the same time, CTX was reacted with Traut's reagent to introduce sulfhydryl groups, mixed with DGL-PEG at a molecular ratio of 1:1, stirred and reacted at room temperature for 24 hours, and unreacted CTX was removed by size exclusion chromatography to obtain DGL-PEG. PEG-CTX.
Embodiment 3
[0079] DGL (second generation) and PEG containing bifunctional groups were dissolved in PBS with pH 8.0 according to the molecular ratio of 1:10, and stirred at room temperature for 2 hours to generate DGL-PEG, and the unreacted PEG was removed by ultrafiltration and the buffer was replaced PBS pH 7.0. At the same time, CTX was reacted with Traut's reagent to introduce sulfhydryl groups, mixed with DGL-PEG at a molecular ratio of 1:1, stirred and reacted at room temperature for 24 hours, and unreacted CTX was removed by size exclusion chromatography to obtain DGL-PEG. PEG-CTX.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com