Metal-boron-nitrogen-hydrogen hydrogen storage material and preparation method thereof
A hydrogen storage material and hydrogen material technology, applied in the field of hydrogen storage technology and new material synthesis, can solve the problems of single type of synthetic material, complex process, difficult to control, etc., and achieve the effect of simple preparation process, simple process and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
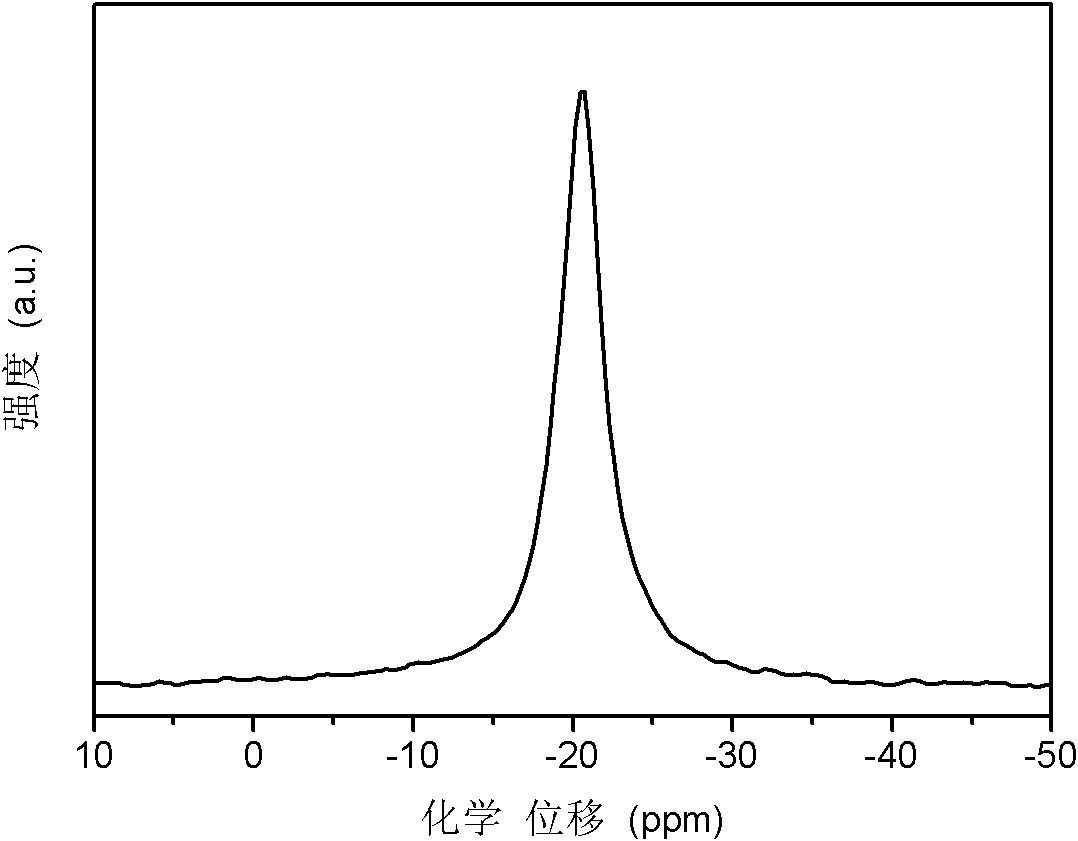
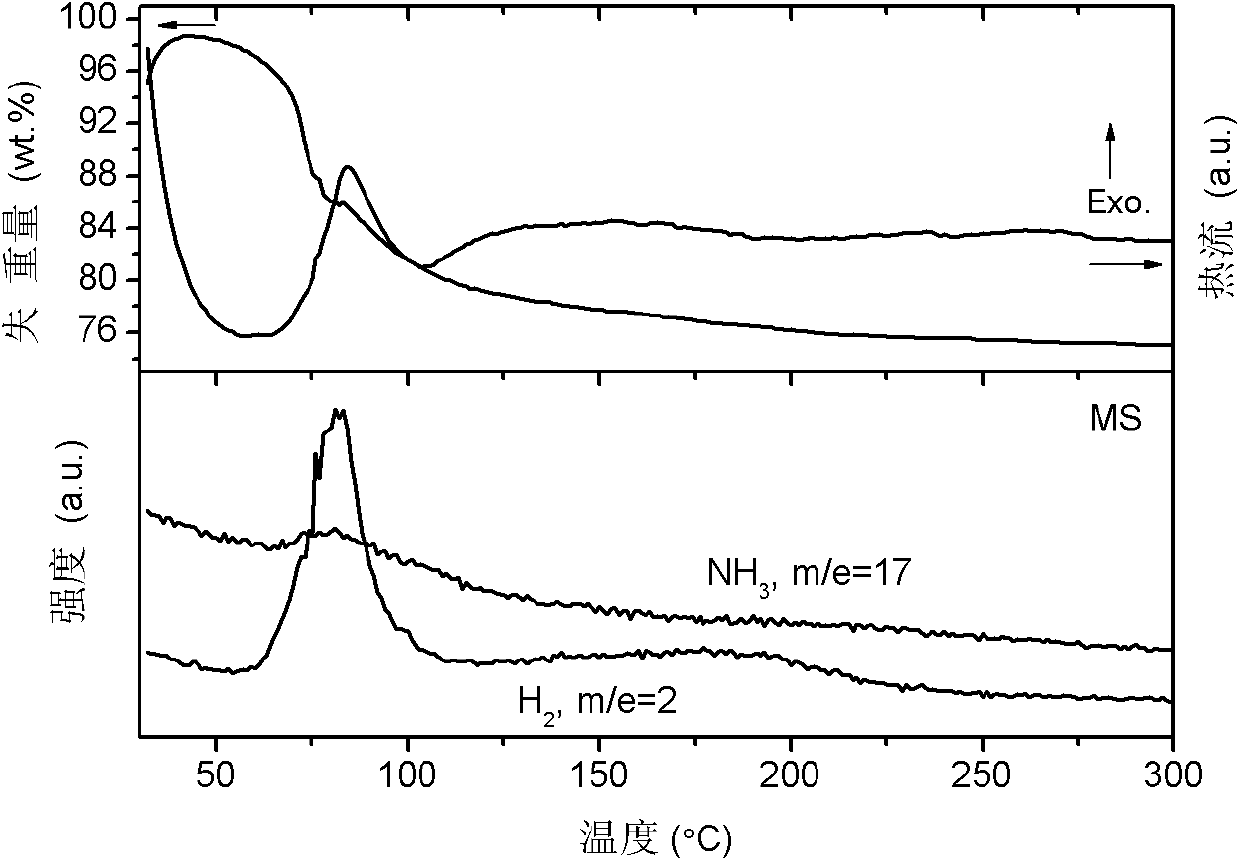
[0030] with 2NH 3 BH 3 +MgH 2 As the starting material, Mg(NH 2 BH 3 ) 2 1.7NH 3 Research on hydrogen storage materials and hydrogen release performance.
[0031] The raw material used is: NH 3 BH 3 (purity 97wt.%), MgH 2 (purity 98wt.%), ammonia (volume purity 99.99%). In an argon atmosphere glove box, a 2:1 molar ratio of NH 3 BH 3 / MgH 2 Put the mixture and stainless steel balls into a stainless steel ball mill tank, cover and seal; then vacuumize the ball mill tank, fill it with ammonia gas for several times, fill it with 2.14bar ammonia gas, and press the molar ratio of NH 3 BH 3 / MgH 2 / NH 3 2:1:1.7; finally, the ball mill jar was placed on a Fritsch 7 planetary ball mill, and ground at a room temperature of 25° C. for 2 hours, with a ball-to-material mass ratio of 40:1.
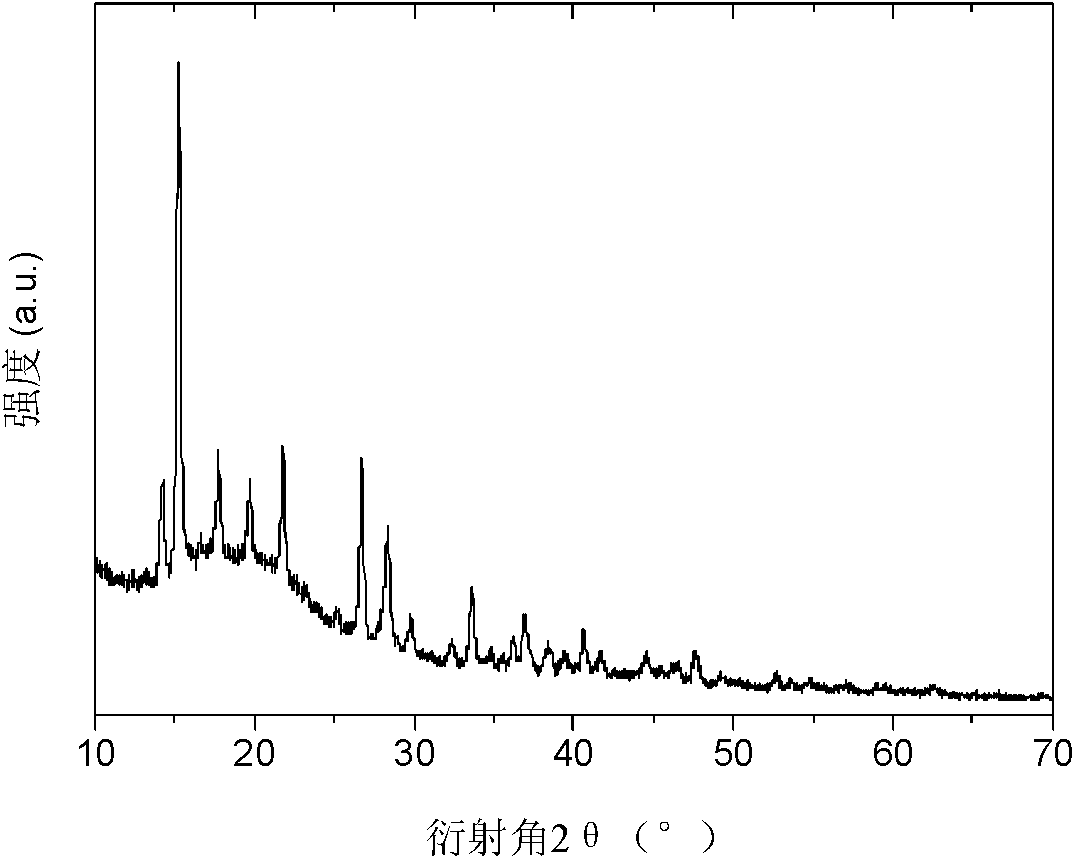
[0032] X-ray testing equipment and conditions: Rigaku D / max 2500, Cu Ka rays. figure 1 The X-ray images of the samples after ball milling are given. NH after ball milling 3 BH 3 and...
Embodiment 2
[0037] with 2NH 3 BH 3 +MgH 2 As the starting material, Mg(NH 2 BH 3 ) 2 1.4NH 3 Hydrogen storage material.
[0038] The raw material used is: NH 3 BH 3 (purity 97wt.%), MgH 2 (purity 98wt.%), ammonia (volume purity 99.99%). Fill the ball mill tank with 1.4 bar of ammonia, according to molar ratio NH 3 BH 3 / MgH 2 / NH 3 The ratio is 2:1:1.5, and the other sample preparation conditions are the same as in Example 1. Figure 5 The X-ray diffraction spectrum of the sample after ball milling is given, and its molecular formula is Mg(NH 2 BH 3 ) 2 1.4NH 3 . and figure 1 The comparison of the results shows that as the amount of ammonia gas in the ball mill tank is reduced, the structure and composition of the generated compound are changed, which shows that the formation of metal ammonia borane can be modulated by changing the amount of reactive ammonia gas The amount of ammonia complexed by the compound can achieve the purpose of modulating the composition and pe...
Embodiment 3
[0040] with 2NH 3 BH 3 +CaH 2 As starting material, Ca(NH 2 BH 3 ) 2 2NH 3 Research on hydrogen storage materials and hydrogen release performance.
[0041] The raw material used is: NH 3 BH 3 (purity 97wt.%), CaH 2 (purity 99.99wt.%), ammonia (volume purity 99.99%). Fill the ball mill tank with 2.2 bar of ammonia, by molar ratio of NH 3 BH 3 / CaH 2 / NH 3 The ratio is 2:1:2.1, and the other sample preparation conditions are the same as in Example 1. Image 6 The X-ray diffraction spectrum of the sample after ball milling is given, and its molecular formula is Ca(NH 2 BH 3 ) 2 2NH 3 . This result is consistent with Chen Ping et al. using the amino salt Ca(NH 2 ) 2 Ca(NH 2 BH 3 ) 2 2NH 3 This shows that the preparation method provided by the present invention has wide applicability, not only can synthesize existing compounds, but also can synthesize metal ammonia borane compounds of many new complex ammonia that cannot be synthesized by other methods, Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com