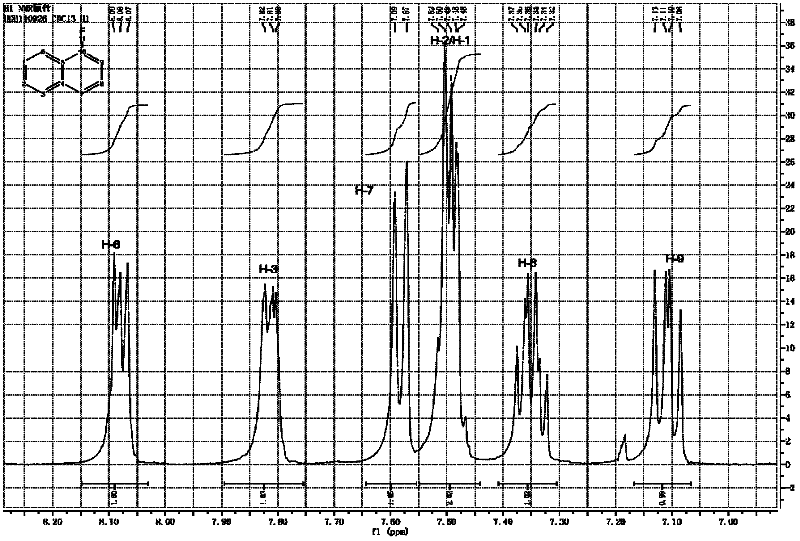
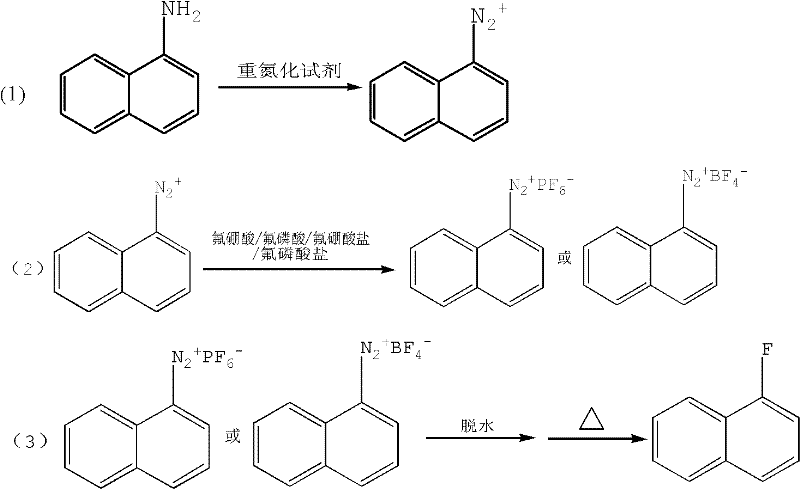
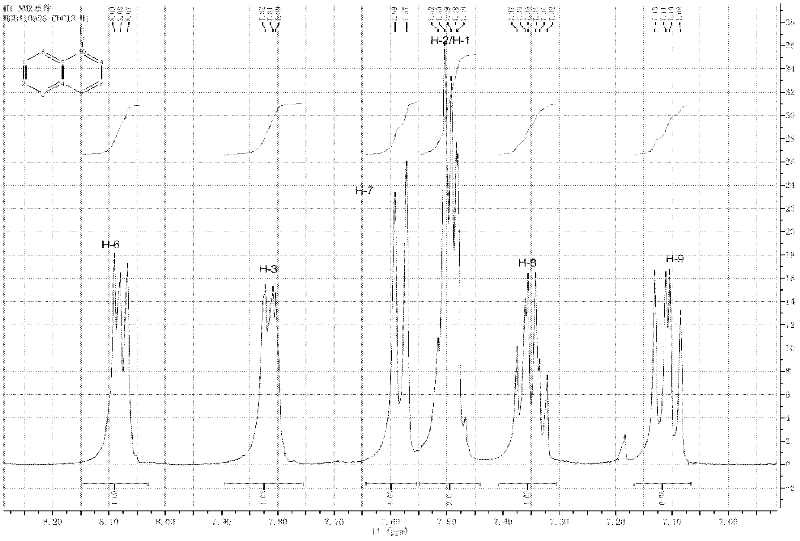
Method for preparing 1-fluoronaphthalene
A technology of fluoronaphthalene and naphthylamine, which is applied in the field of preparation of the key intermediate 1-fluoronaphthalene, can solve problems such as many influencing factors, difficulty in realizing industrial production, harsh reaction conditions, etc., and achieve the goal of improving product yield and safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of diazonium fluoroborate
[0030] Put 225g of 30% HCl into a 1000ml four-necked bottle, slowly add 72g (0.5mol) of 1-naphthylamine, then cool to -10°C, add 85g of ice, 144ml of water, and then drop in sodium nitrite solution (sodium nitrite 41.4g+ Prepared with 60ml of water), the dropwise addition process ensures that the temperature is controlled at -5-0°C, after the addition is complete, stir for 30 minutes.
[0031] Slowly add 176g of 40% fluoboric acid dropwise. During the dropwise addition, the viscosity increases. After the dropwise addition, stir for 15 minutes, filter and dry, rinse with ice methanol 100ml three times, and then filter dry. The solid weighs 115g. Return the solid to a dry four-necked flask, add 150ml of toluene, stir for 30min, and filter with suction. Then beat with 100 ml of toluene, stir and filter until dry, and weigh 110 g of the obtained diazonium fluoroborate (moisture content 0.1%, toluene content 1.0%). ...
Embodiment 2
[0032] Embodiment 2: the preparation of diazonium fluoroborate
[0033] According to Example 1, the amount of 40% fluoboric acid is reduced to 143g, the solid viscosity is too large, and it is difficult to stir. After suction filtration to dryness, the obtained diazofluoroborate weighs 95g (moisture content 0.25%, Toluene content 1.5%).
Embodiment 3
[0034] Embodiment 3: the preparation of diazonium fluoroborate
[0035] According to Example 1, sulfuric acid was used instead of hydrochloric acid, and the obtained diazonium fluoroborate weighed 102 g (moisture content 0.2%, toluene content 1.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com