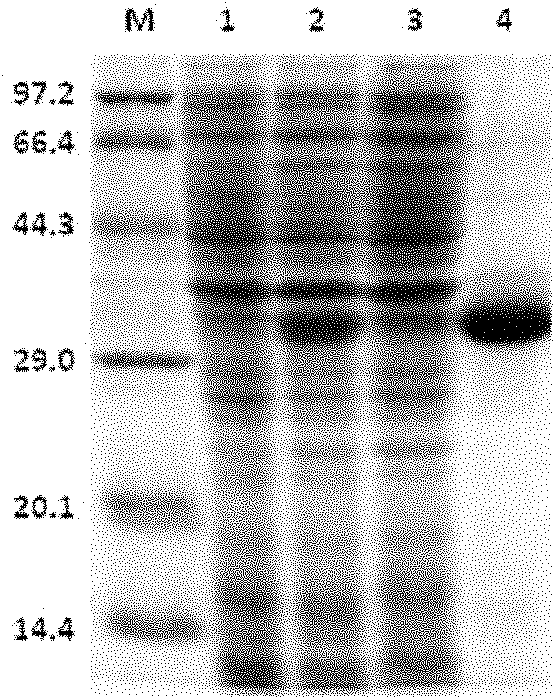
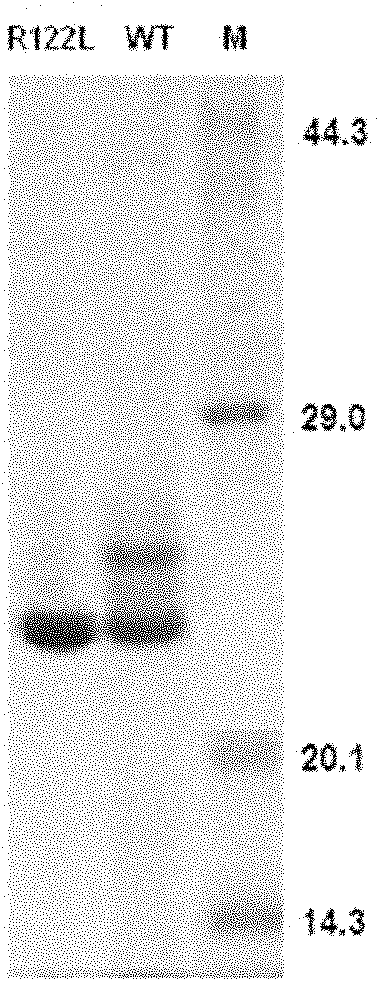
Human anionic trypsin mutant
A technology of trypsin and anion, applied in the direction of enzymes, using carriers to introduce foreign genetic material, enzymes, etc., can solve the problems of low activity and poor stability of natural enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1, mutation site
[0082] The amino acid sequence of wild-type human anionic trypsinogen (hTg2) is as follows (SEQ ID NO: 2):
[0083] M N L L L I L T F V A A A V A A P F D D D D K I V G G Y I C E E N S V P Y Q V S L N S G Y H F C G G S L I S E Q W V V S A G H C Y R I Q V R L G E H N I E V L E G N E Q F I N A A K I I R H P K Y N S R T L D N D I L L I K L S S P A V I N S S A I S L P T A P P A A G T E S L I S G W G N T L S S G A D Y P D E L Q C L D A P V L S Q A E C E A S Y P G K I T N N M F C V G F L E G G K D S C Q G D S G G P V V S N G E L Q G I V S W G Y G C A Q K N R P G V Y T K V Y N Y V D W I K D T I A A N S
[0084] The nucleotide sequence of wild-type human anionic trypsinogen (hTg2) is as follows (SEQ ID NO: 1):
[0085] GCTCCGTTTGACGATGACGACAAAATCGTGGGTGGTTACATCTGCGAGGAGAACTCCGTGCCGTACCAGGTTTCTCTCAATTCTGGCTACCACTTCTGCGGTGGCAGCCTGATCTCTGAACAGTGGGTAGTGTCTGCTGGCCATTGCTACAAGTCTCGTATCCAGGTACGTCTGGGTGAACACAACATCGAAGTACTCGAAGGCAACGAGCAGTTCATCAACGCT...
Embodiment 2
[0091] Embodiment 2, the expression of mhTg2 (R122L)
[0092] Add HindIII and BamHI restriction sites to both ends of the DNA sequence of the mutant obtained above, and insert the mutant DNA into the HindIII / BamHI site of the pET-32a expression vector after HindIII / BamHI digestion, and obtain The recombinant vector is called pET-32a-R122L.
[0093] In order to serve as a control, the present inventors also added HindIII and BamHI restriction sites to both ends of the DNA sequence of the wild-type human anionic trypsinogen (hTg2), respectively, and inserted the mutant DNA into Into the HindIII / BamHI site of the pET-32a expression vector, the obtained recombinant vector is called pET-32a-hTg2.
[0094] The recombinant vectors pET-32a-R122L and pET-32a-hTg2 were respectively transformed into competent Escherichia coli DH5α cells to obtain transformants, and the correctly inserted recombinant expression vectors were obtained by sequencing.
[0095] Pick the monoclonal colony on ...
Embodiment 3
[0097] Embodiment 3, the purification of mhTg2 (R122L)
[0098] The inclusion bodies of recombinant mhTg2(R122L) or hTg2 were refolded and activated to obtain active mhT2(R122L) mutein or hTg2 protein, respectively. The cell suspension was ultrasonically disrupted, and centrifuged at 12000 rpm for 10 min at low temperature. The supernatant was discarded, and the precipitate was fully washed with 0.5% Triton X-100 solution and 20mM Tris-HCl pH 8.0 buffer respectively. After dissolving the washed inclusion body with 8M Urea solution, slowly add it to the refolding solution (50mMpH8.0Tris-HCl, 10mM GSH, 5mM GSSG) for dilution and refolding, after 12h, add enterokinase (enterokinase: Recombinant protein) (w:w)=1:100) was activated for 2 hours to obtain activated trypsin.
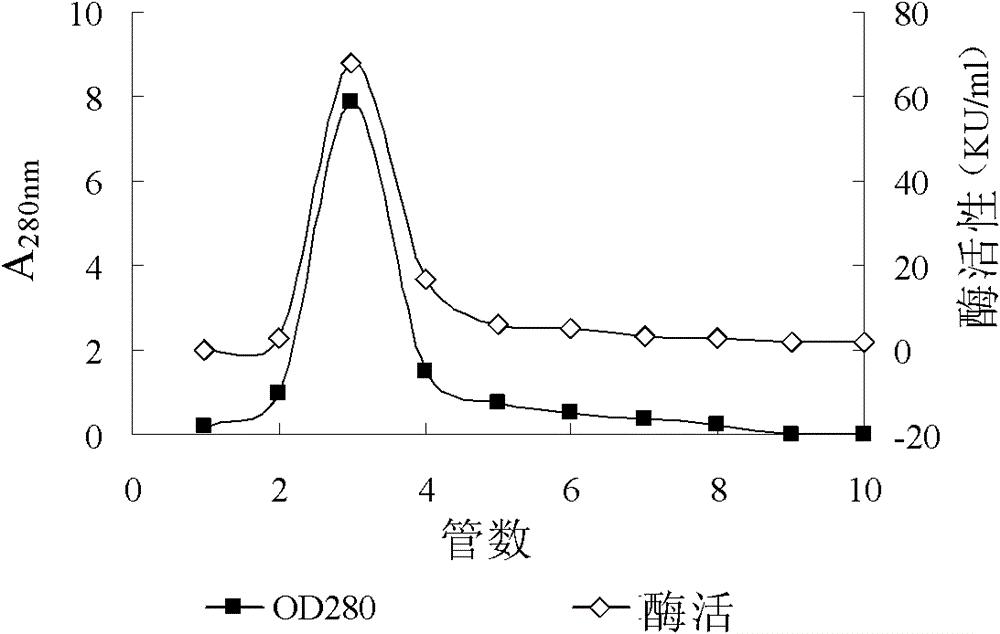
[0099] Put the obtained active protein on the column, the equilibration and elution conditions are as follows: use a 2×15cm chromatographic column, load an appropriate amount of CM-FF cation exchange resin, eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com