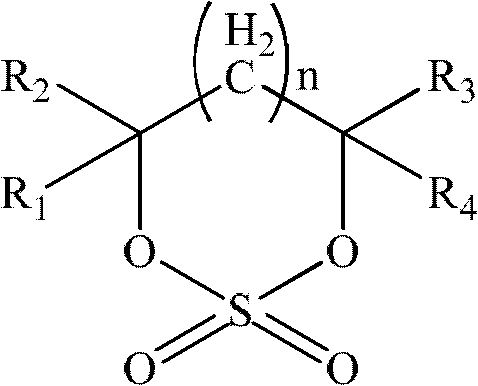
Non-aqueous electrolyte for lithium ion battery and lithium ion secondary battery
A non-aqueous electrolyte and lithium-ion battery technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of γBL-based electrolyte capacity attenuation, high-temperature storage performance degradation, etc., achieve excellent high-temperature storage performance and reduce reaction properties, and the effect of improving high-temperature storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] One. The preparation method of embodiment electrolyte
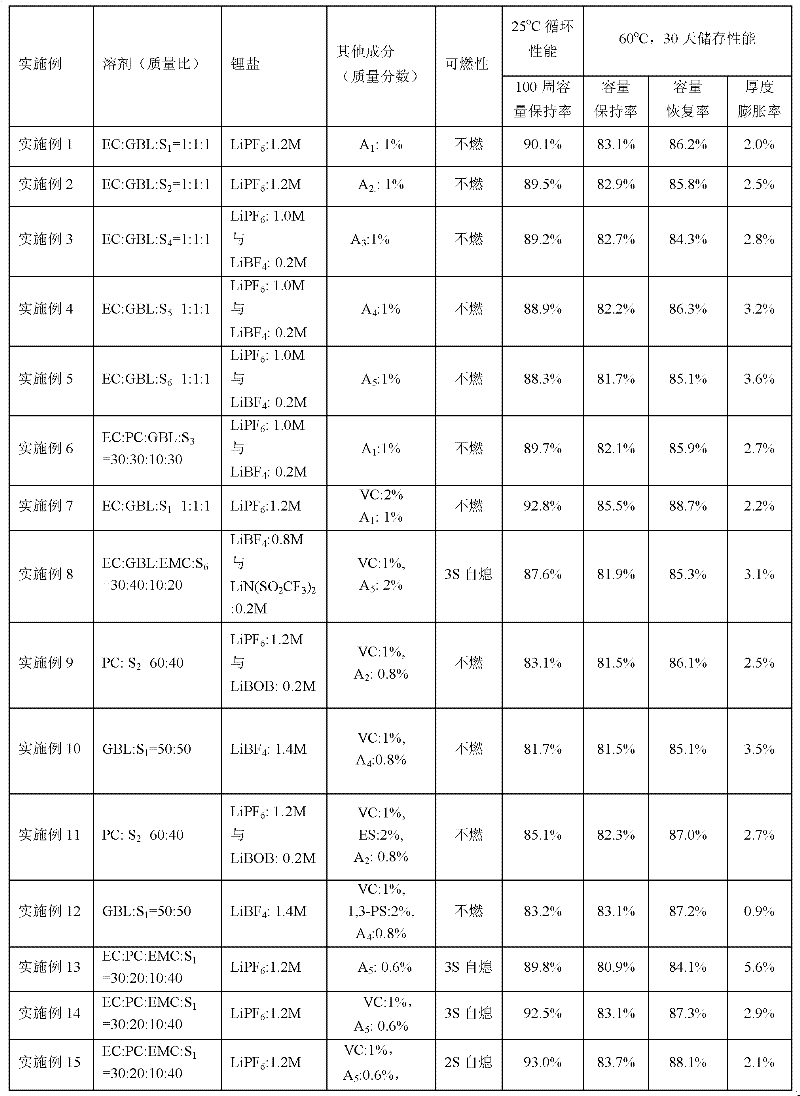
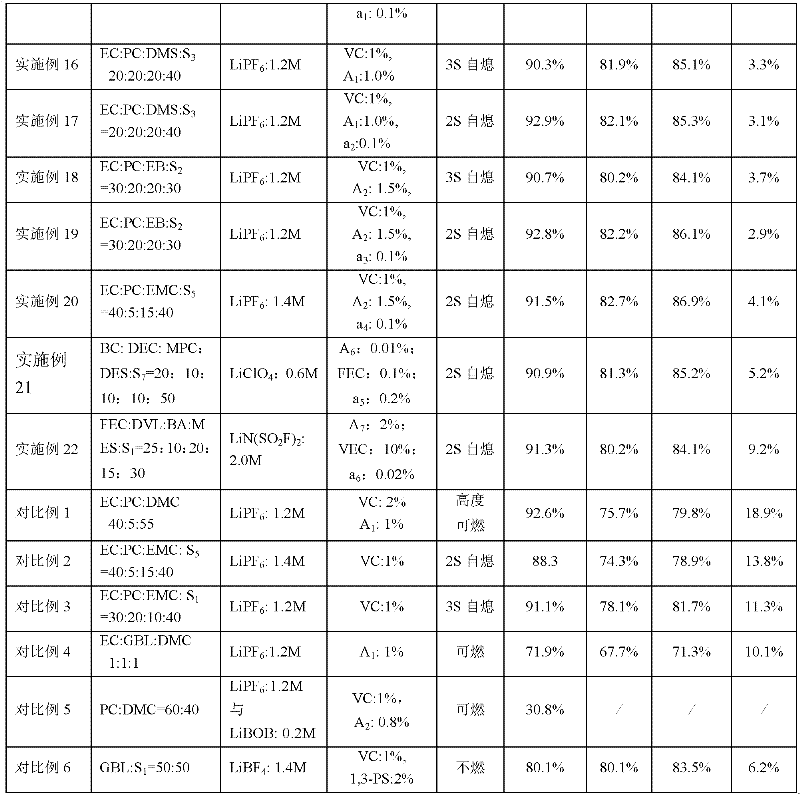
[0041] in an argon-filled glove box (H 2 (0<10ppm), cyclic carbonate, cyclic carboxylate, linear fluoroether, lithium salt, cyclic sulfuric acid ester, film-forming additive and fluorocarbon surfactant are listed according to each embodiment and comparative example of table 1 The electrolyte mass ratio is prepared. The above-mentioned raw materials are added in sequence and fully stirred evenly to obtain the lithium-ion battery electrolyte of the present invention, which is used for flammability test and battery performance test.
[0042] Two. The manufacture method of embodiment lithium-ion battery
[0043] The non-aqueous electrolyte secondary battery of the present invention is composed of the above-mentioned non-aqueous electrolyte, a negative electrode and a positive electrode.
[0044] The active material constituting the positive electrode can be LiCoO 2 , LiMn 2 o 4 , LiNi 1-x-y co x mn y o 2 (01-...
Embodiment 1
[0089] in an argon-filled glove box (H 2 O1 = 1:1:1 mixed with lithium hexafluorophosphate (1.2M), the additive is cyclic sulfate A 1 , accounting for 1% of the total weight of the electrolyte. The above-mentioned raw materials are added in sequence, fully stirred evenly, and the lithium-ion battery electrolyte solution (free acid<30ppm, moisture<10ppm) of the present invention is obtained. Electrolyte is used for flammability test and battery performance test. The test results of flammability and the capacity retention rate at the 100th cycle of normal temperature cycle; the capacity retention rate, capacity recovery rate and thickness expansion rate after storage at 60°C for 30 days are shown in Table 1.
Embodiment 2
[0091] The same as the process of Example 1, the difference is that the fluoroether is S 2 , the cyclic sulfate is A 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com