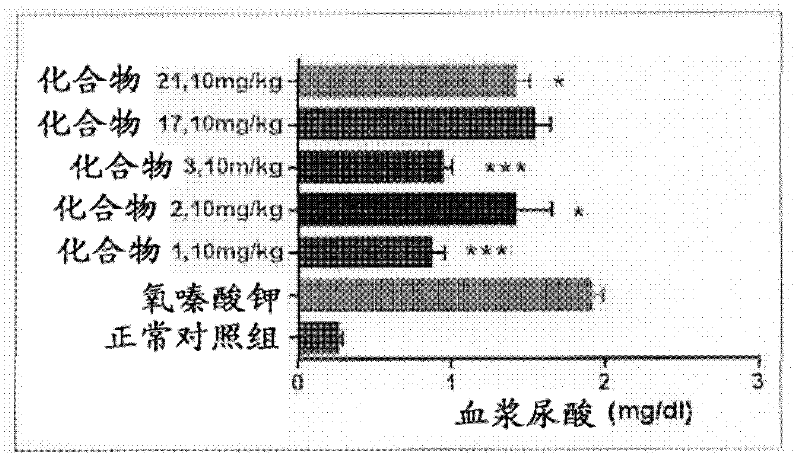
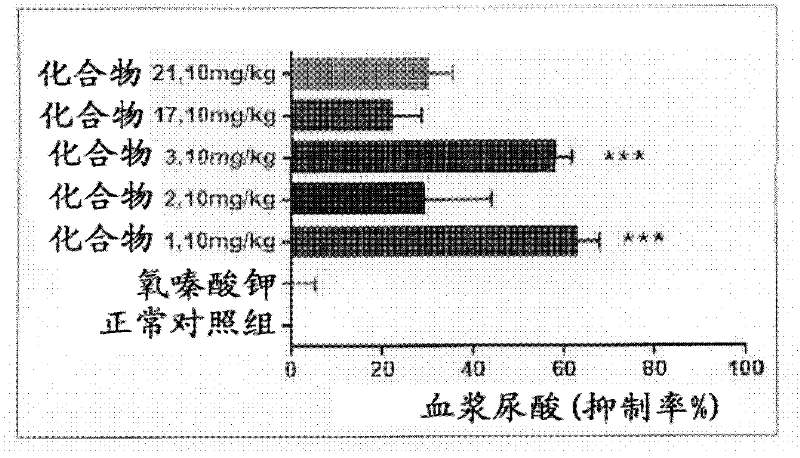
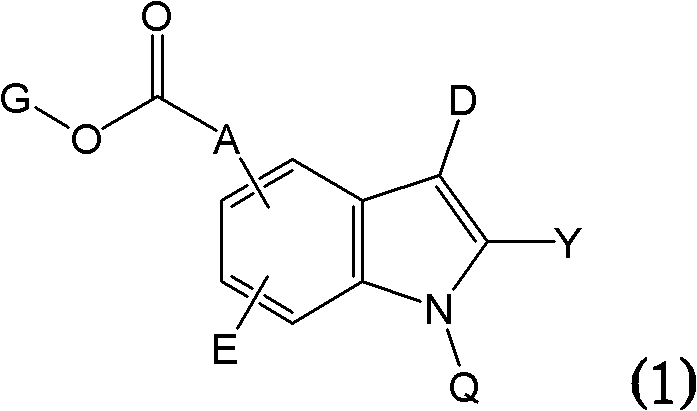
Novel compounds effective as xanthine oxidase inhibitors, method for preparing the same, and pharmaceutical composition containing the same
一种化合物、药学的技术,应用在有效作为黄嘌呤氧化酶抑制剂的新化合物、该化合物的制备和含有该化合物的药物组合物领域,能够解决致命、不可预测、差顺应性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0212] Preparation Example 1: Synthesis of ethyl 1-(1H-indol-5-yl)pyrazole-4-carboxylate
[0213]
[0214] 1H-Pyrazole-4-carboxylic acid ethyl ester (1.00 g, 7.14 mmol) and 1H-indol-5-ylboronic acid (1.15 g, 7.14 mmol) were dissolved in N,N-dimethylformamide (70 mL) middle. Copper(II) acetate (0.972 g, 5.35 mmol) and pyridine (1.2 mL, 14.8 mmol) were added, and the mixture was stirred at room temperature for 3 days. The solvent was distilled off under reduced pressure, and the residue was purified by column chromatography to obtain the title compound (1.40 g, 5.47 mmol, 77% yield).
[0215] NMR: 1 H-NMR (CDCl 3)δ8.39(1H, s), 8.30(1H, br), 8.11(1H, s), 7.92(1H, d), 7.54(1H, dd), 7.47(1H, d), 7.31(1H, t ), 6.64-6.62 (1H, m), 4.35 (2H, q), 1.39 (1H, t)
[0216] Mass spectrum (EI) 256 (M + +1)
preparation example 2
[0217] Preparation 2: Synthesis of ethyl 1-(3-cyano-1H-indol-5-yl)pyrazole-4-carboxylate The title compound was obtained by the following steps (1), (2) and (3) .
[0218] (1) Synthesis of ethyl 1-(3-formyl-1H-indol-5-yl)pyrazole-4-carboxylate
[0219]
[0220] Oxalyl chloride (0.56mL, 6.6mmol) was added into anhydrous dichloromethane (50mL), N,N-dimethylformamide (0.51mL, 6.6mmol) was added thereto at 0°C, and the mixture was heated at 0°C Stir for 30 minutes. To the reaction solution was added a mixture of ethyl 1-(1H-indol-5-yl)pyrazole-4-carboxylate (1.40 g, 5.47 mmol) obtained in Preparation Example 1 and dichloromethane (50 mL). The mixture was stirred at room temperature under reflux for 1 hour, and the solvent was removed. Tetrahydrofuran (100 mL) and 20% aqueous ammonium acetate (100 mL) were added, then it was heated for 30 minutes while stirring at reflux. After the reaction, the reaction solution was cooled. Ethyl acetate was added, and the mixture was wash...
preparation example 3
[0231] Preparation Example 3: Synthesis of ethyl 1-(3-cyano-1-isopropyl-indol-5-yl)pyrazole-4-carboxylate
[0232]
[0233] Ethyl 1-(3-cyano-1H-indol-5-yl)pyrazole-4-carboxylate (13.84 g, 49.38 mmol) obtained in Preparation 2 was dissolved in acetonitrile (200 mL). Cesium carbonate (32.17 g, 98.74 mmol) and 2-iodopropane (19.7 mL, 198 mmol) were added, then heated for 5 hours while stirring at reflux. After the reaction, the reaction solution was concentrated under reduced pressure, and the obtained solid was purified by column chromatography to obtain the title compound (13.87 g, 43.03 mmol, 87% yield).
[0234] NMR: 1 H-NMR (CDCl 3 )δ8.48(1H, s), 8.16(1H, s), 8.06(1H, d), 7.82(1H, s), 7.78(1H, dd), 7.57(1H, d), 4.80-4.73(1H , m), 4.38 (2H, q), 1.64 (6H, d), 1.42 (3H, t)
[0235] Mass spectrum (EI) 323 (M + +1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com