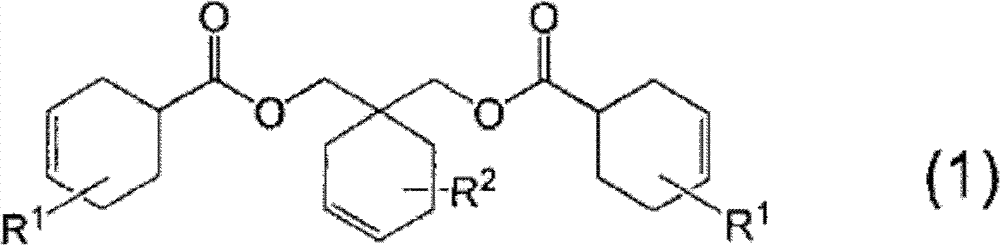
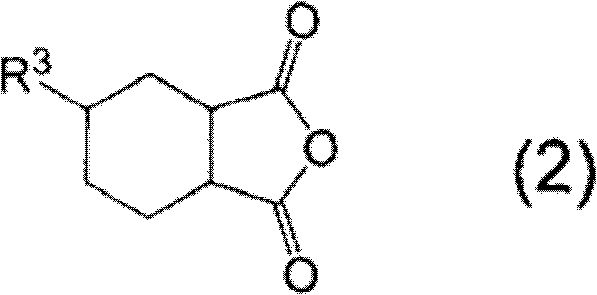
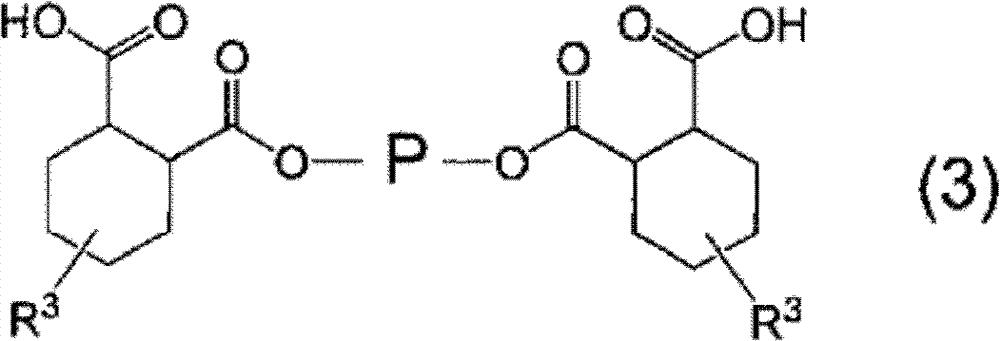
Curable resin composition and cured products thereof
A technology for curing resins and compositions, applied in the fields of electric solid devices, semiconductor/solid-state device manufacturing, semiconductor/solid-state device components, etc., can solve the problems of decreased illumination and residual durability of LED products, and achieve excellent corrosion resistance. , The effect of preventing sag and excellent coloring resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0174] Hereinafter, the present invention will be described more specifically through examples. In the following description, unless otherwise specified, "parts" means parts by weight. In addition, the present invention is not limited to these Examples. In addition, in an Example, epoxy equivalent was measured based on JIS K-7236, and viscosity was measured using the E-type viscometer at 25 degreeC. In addition, the analysis conditions of gas chromatography (hereinafter referred to as GC) are: HP5-MS (0.25mm I.D.×15m, film thickness 0.25μm) is used for the separation column, the initial temperature of the column oven temperature is set to 100°C, and the The temperature was raised at a rate of 15°C and held at 300°C for 25 minutes. In addition, helium was used as a carrier gas. In addition, the measurement conditions of gel permeation chromatography (hereinafter referred to as GPC) are as follows. The column uses ShodexSYSTEM-21 column (KF-803L, KF-802.5 (×2 pieces), KF-802)...
Synthetic example 1
[0176] Synthesis of Cyclohexene Diol
[0177] Synthesis example 1 (refer to patent document EP 0487035B1)
[0178] In a flask with a stirrer, a reflux condenser, and a stirring device, 112 parts of cyclohexene formaldehyde, 600 parts of ethanol, 300 parts of 35% formalin, and 284 parts of 30% by weight potassium carbonate aqueous solution were added while purging nitrogen. Parts, while stirring, the temperature was raised to reflux temperature, and the reaction was carried out in this state for 9 hours. After completion of the reaction, a Dean-Stark condenser was installed, and the bath temperature was kept at 100° C. for 4 hours to distill off ethanol. After cooling to room temperature, let stand as it is for 24 hours. Cyclohexene dimethanol deposited as white crystals was filtered out from the solution by filtration under reduced pressure and dried to obtain 103 parts of the target cyclohexanediol (the following formula (6)). Gas chromatographic purity was obtained in 98 ...
Synthetic example 2
[0181] In a flask with a stirrer, a reflux condenser, a stirring device, and a Dean-Stark tube, 150 parts of toluene, 70 parts of a compound of the formula (6), 3-cyclohexane 126 parts of olefinic acid and 2 parts of p-toluenesulfonic acid were reacted for 10 hours while heating under reflux while removing water. After completion of the reaction, wash twice with 50 parts of 10% by weight aqueous sodium bicarbonate solution, then wash twice the organic layer obtained with 50 parts of water, then concentrate the organic solvent with a rotary evaporator, thus obtaining the alkene compound of the present invention ( D-1, following formula (7)) 173 parts. The obtained olefin compound was in a liquid state, and its purity as measured by gas chromatography was 92 area%, and as a result of analysis by gel permeation chromatography, it was confirmed that the purity was >98 area%.
[0182]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com