Preparation method for slow-release tablet of indapamide-containing medicament
A technology for indapamide and sustained-release tablets, which is applied in the field of preparing a sustained-release preparation containing indapamide medicines, can solve the problem of increased difficulty in preparation quality control, increased cost and prescription complexity, and easy re-partitioning of compressed tablets. layer and other problems, to achieve the effect of simple and easy production process, reduced mutual influence and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] In an embodiment, a method for preparing a sustained-release tablet containing indapamide, comprising the following steps:
[0019] 1) Wetting: A conventional wet granulator with an atomizing device is used, and the purified water enters the mixing tank of the closed wet granulator in an atomized form through the atomizing device. At the same time, the hydroxypropyl methylcellulose Add the prime (HPMC) powder into the mixing tank, and keep stirring to make the surface of the HPMC powder fully contact with the water mist to obtain the HPMC wet material;
[0020] 2) Granulation: Use a swinging granulator to granulate the HPMC wet material obtained in step 1), and the obtained granules are dried and sized to obtain HPMC granules.
[0021] 3) Mixing: uniformly mix the HPMC granules obtained in step 2) with indapamide and lubricant in a weight ratio of 50-100:1.5:0.25-1, and compress the mixture into tablets to obtain indapamide sustained-release tablets.
[0022] In the em...
Embodiment 1
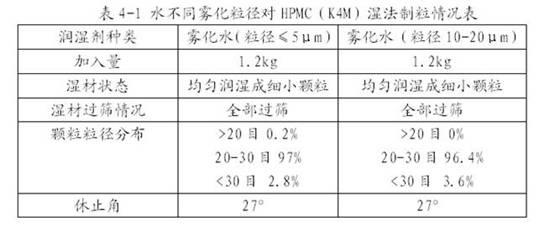
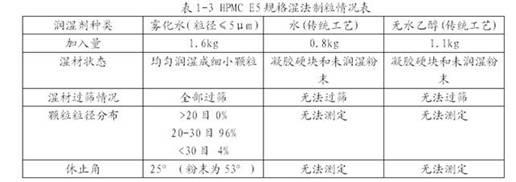
[0027] Compared the results of wet granulation of HPMC E5 (5 mPa s), K4M (4000mPa s) and K100M (100000mPa s) with different viscosities by using the granulation method of the present invention and directly adding water and adding absolute ethanol. It can be seen that adopting the method of the present invention can produce granules with good appearance and fluidity for HPMC with different viscosities, and adding more water can also improve the viscosity of the material and enable normal granulation. However, directly adding water or absolute ethanol to granulate high and low viscosity HPMC cannot uniformly wet it. Even if a small amount is added, gel "pimples" of varying degrees will be formed rapidly, and granules cannot be obtained by sieving.
[0028] Add 10kg of HPMC with different viscosity specifications into the high-efficiency wet granulator, add atomized water (particle size ≤ 5μm), non-atomized water and absolute ethanol at a speed of 60g / min, and granulate. The part...
Embodiment 2
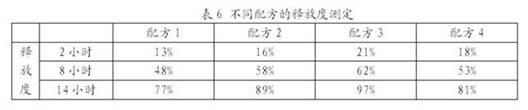
[0034] Compared the results of wet granulation of the mixture containing 30% HPMC (K4M) and 70% lactose as diluent by directly adding water and adding absolute ethanol by using the granulation method of the present invention, it can be known from the results that by using this method, Granules with good appearance and flow properties can be obtained, and the granulation process is easier to carry out than in Example 1. By directly adding absolute ethanol, it can basically be evenly wetted, with only a small amount of gel "pimples", most of which can be sieved to obtain better particles. If water is added directly, severe gel "pimples" are still formed, and granules cannot be obtained by sieving. The granules made with absolute ethanol are looser, brittle when granulated, and have more fine powders.
[0035] Add 10 kg of K4M HPMC and lactose (3:7) mixture into the high-efficiency wet granulator, and add atomized water (particle size ≤ 5 μm), non-atomized water and absolute eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com