Surfactant and preparation method thereof
A technology of surfactant and surfactant, applied in the field of surfactant and its preparation, can solve the problems of reduced biological activity or interface activity, waste of chemical agents, and non-single product, so as to reduce charge density and improve biological activity and surface activity, the effect of pore film structure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
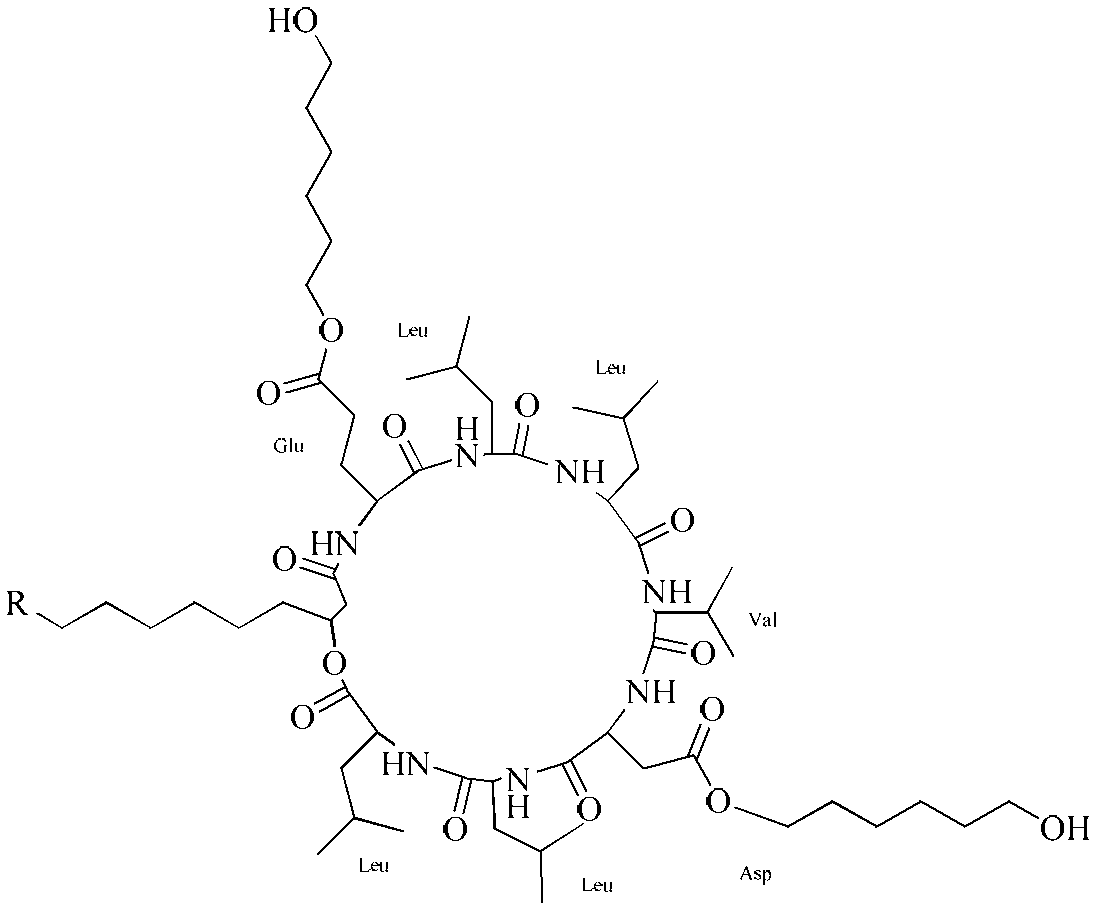
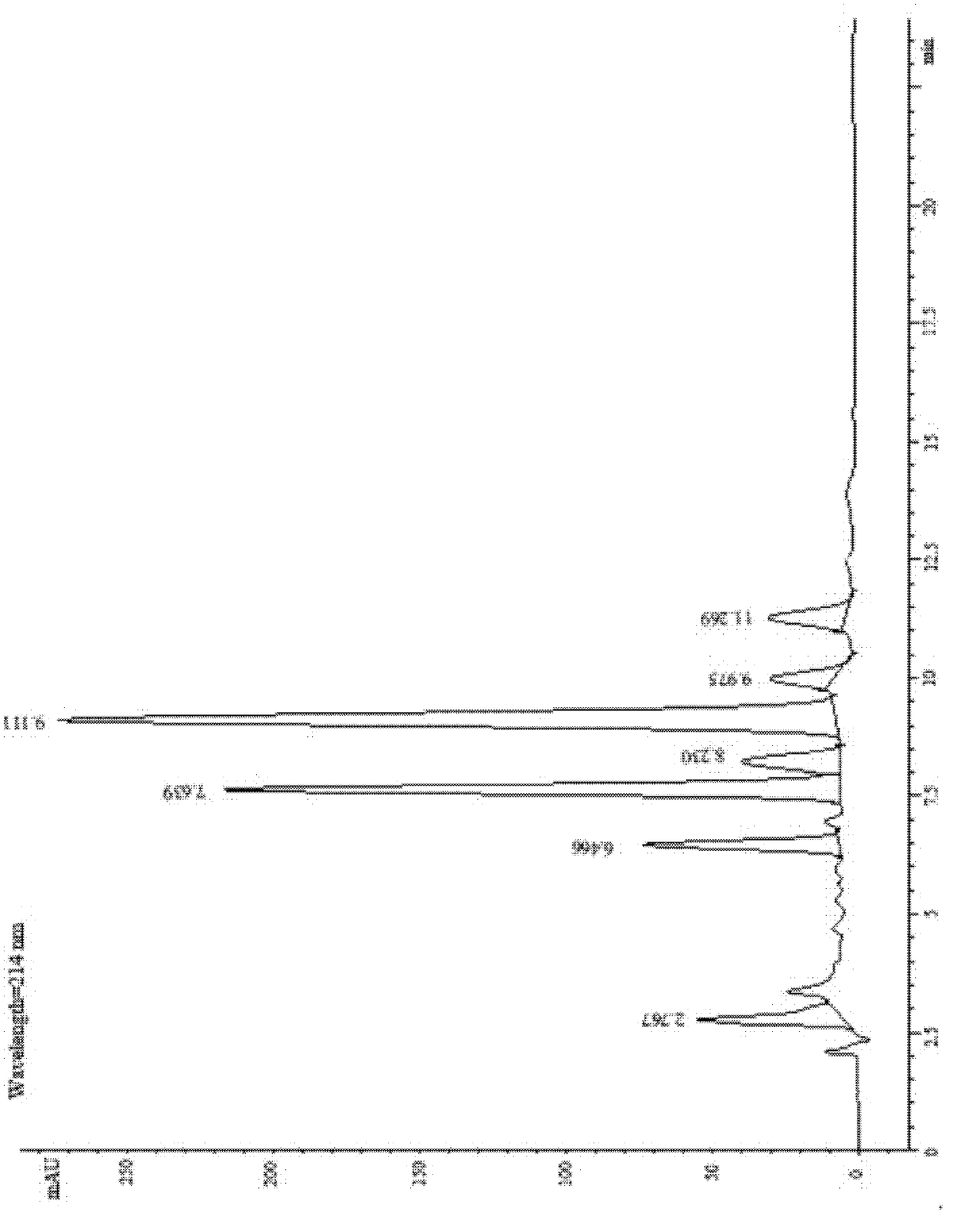
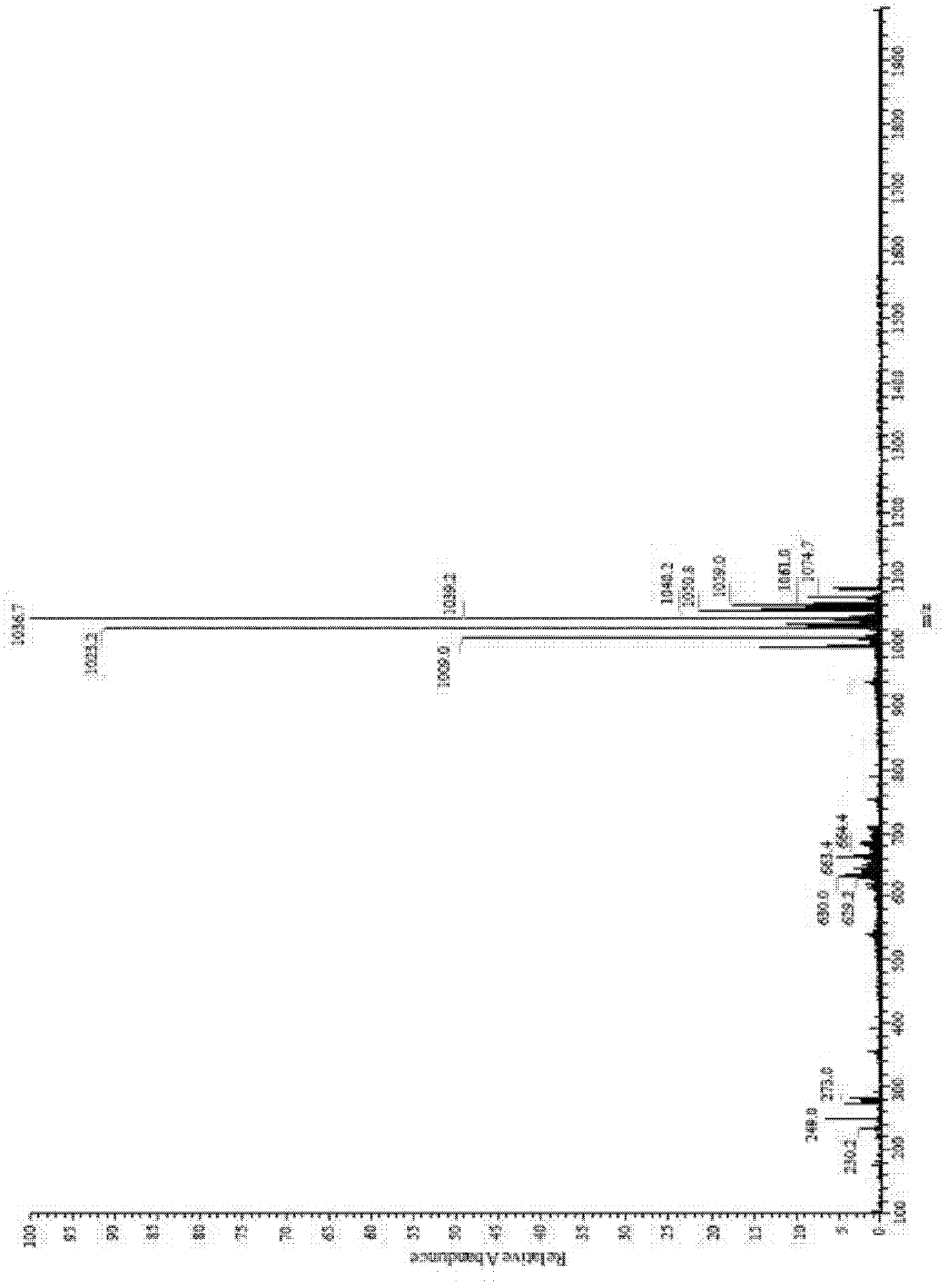
[0032] In 100mL concentration of 10g / L surfactin (surfactin) in chloroform solution, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) 0.77g while stirring , 4-dimethylaminopyridine (DMAP) 0.049g, 1,6-hexanediol 0.48g (the amount of EDC, DMAP and 1,6-hexanediol is 4 times the molar amount of surfactin). After the feeding was completed, the reaction was stirred at 35° C. for 14 h. After the reaction finished, adopt preparative high performance liquid chromatography separation and purification product surfactin-Glu-, Asp-(6-hydroxyl-hexyl) diester (see figure 1 ), the yield is 97.6%.
Embodiment 2
[0034] In the dichloromethane solution that 100mL concentration is 8g / L surfactin (surfactin), add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) 0.46g while stirring , 4-dimethylaminopyridine (DMAP) 0.029g, 1,6-hexanediol 0.28g (the amount of EDC, DMAP and 1,6-hexanediol is 3 times the molar amount of surfactant). After feeding, the reaction was stirred at 25°C for 19h. After the reaction, the product surfactin-Glu-, Asp-(6-hydroxyl-hexyl) diester was separated and purified by preparative high-performance liquid chromatography, and the yield was 97.4%.
Embodiment 3
[0036] In 100mL concentration of 5g / L surfactant (surfactin) tetrahydrofuran solution, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) 0.19g while stirring, 4 -0.012g of dimethylaminopyridine (DMAP) and 0.12g of 1,6-hexanediol (the amount of EDC, DMAP and 1,6-hexanediol is 2 times of the molar amount of surfactin). After feeding, the reaction was stirred at 18°C for 24h. After the reaction, the product surfactin-Glu-, Asp-(6-hydroxyl-hexyl) diester was separated and purified by preparative high-performance liquid chromatography, and the yield was 97.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com