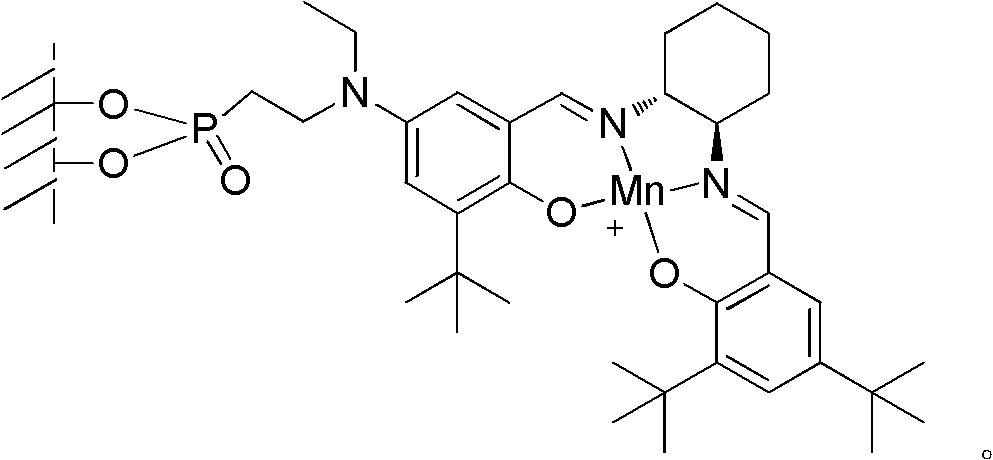
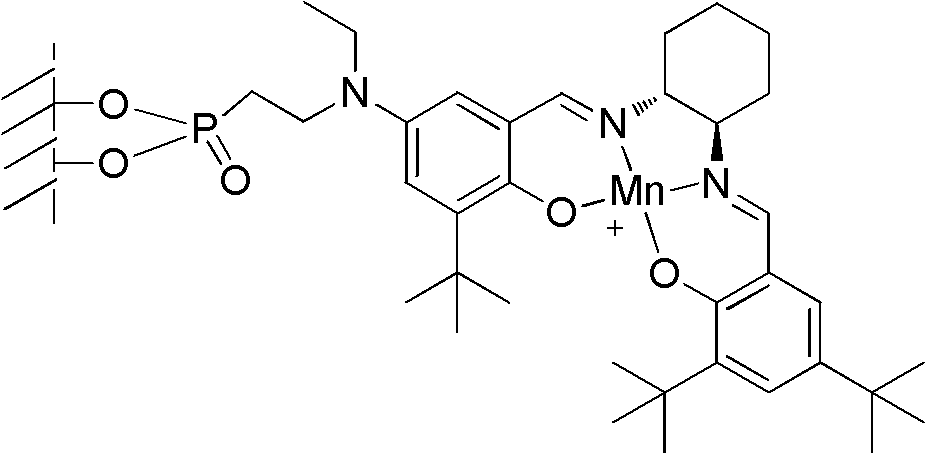
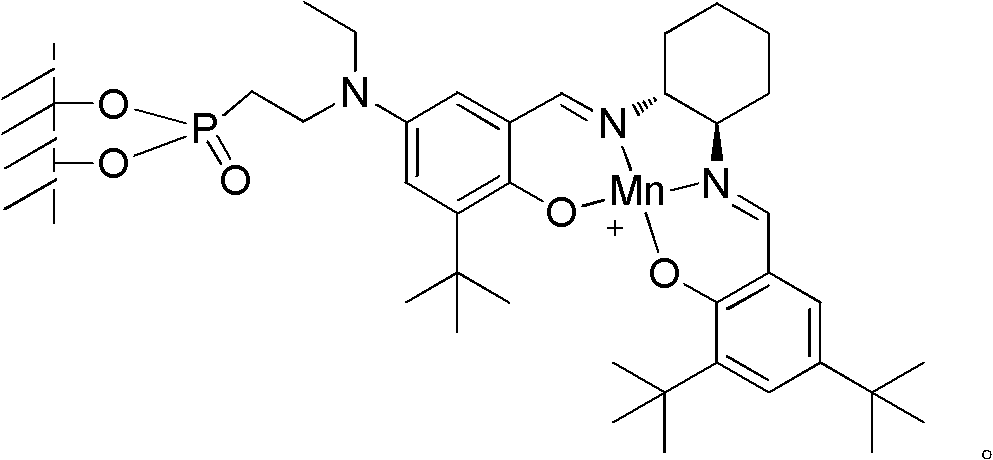
Loaded type salen manganese complex catalyst, preparation method and application thereof
A manganese complex, supported technology is applied to the supported salen manganese complex catalyst and the field of preparation and application thereof, which can solve the problems of only 20%, high cost, low catalytic activity, etc. Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, a kind of preparation method of loaded type salen manganese complex catalyst, carries out following steps successively:
[0043] 1), γ-alumina surface treatment:
[0044] A. Add 10g of γ-alumina and 0.2g of sodium to 50mL of toluene, stir and reflux for 1.5 hours;
[0045] B. Cool the mixed solution obtained in step A to 5°C, add 1g of 2-chloroethylphosphonyl chloride in 10mL toluene solution dropwise, drop it in about 15 minutes, continue to stir at 5°C for 2 hours after the drop, filter, and the obtained filter Cake I was washed with clear water and then dried (50°C, dried to constant weight), to obtain filter cake I after drying;
[0046] C, filter cake I and 30g ethylamine are reacted 4 hours under the MPa pressure of 0.1~0.5 in autoclave after drying, reaction temperature 50~55 ℃;
[0047] After the reaction is completed, cool down to room temperature, filter, wash the filter cake II with water and then dry (50°C, dry to constant weight); obtain the...
Embodiment 2
[0055] The epoxidation of embodiment 2, styrene
[0056] Add 10 mL of water, 1 mmol of styrene, 0.08 mmol of pyridine N-oxide and 50 mg of the supported salen manganese complex catalyst prepared in Example 1 (that is, about 0.02 mmol of the Salen manganese complex) into the reaction kettle, and slowly drop Add 1.5 mmol of hydrogen peroxide with a mass concentration of 15%, drop it in about 1 hour, and continue the reaction at 0° C. for 5 hours after the drop.
[0057] The obtained reaction product was separated and processed by column chromatography to obtain 120 mg of styrene oxide with a purity of 99%.
[0058] The yield of styrene oxide was 99%, and the ee value was 72%.
Embodiment 3
[0059] The epoxidation of embodiment 3, cyclohexene
[0060] Add 10mL of water, 1mmol of cyclohexene, 0.08mmol of pyridine N-oxide and 50mg of the loaded salen manganese complex catalyst prepared in Example 1 in the reaction kettle, and slowly add 1.5mmol of a 25% mass concentration at 0°C Hydrogen peroxide, drop in about 1 hour, and continue to react at 0°C for 6 hours after the drop.
[0061] The obtained reaction product was separated and processed by column chromatography to obtain 95 mg of epoxycyclohexane with a purity of 99%.
[0062] The yield of epoxycyclohexane was 96%, and the ee value was 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com