Preparation method for 4-substituted acylamino cyclohexanone
A technology of amido and cyclohexanone, which is applied in the field of preparation of 4-substituted amido cyclohexanone, can solve the problems of cumbersome process operation, environmental pollution, high cost, etc., and achieve high yield, reasonable route and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 4-acetamidocyclohexanone
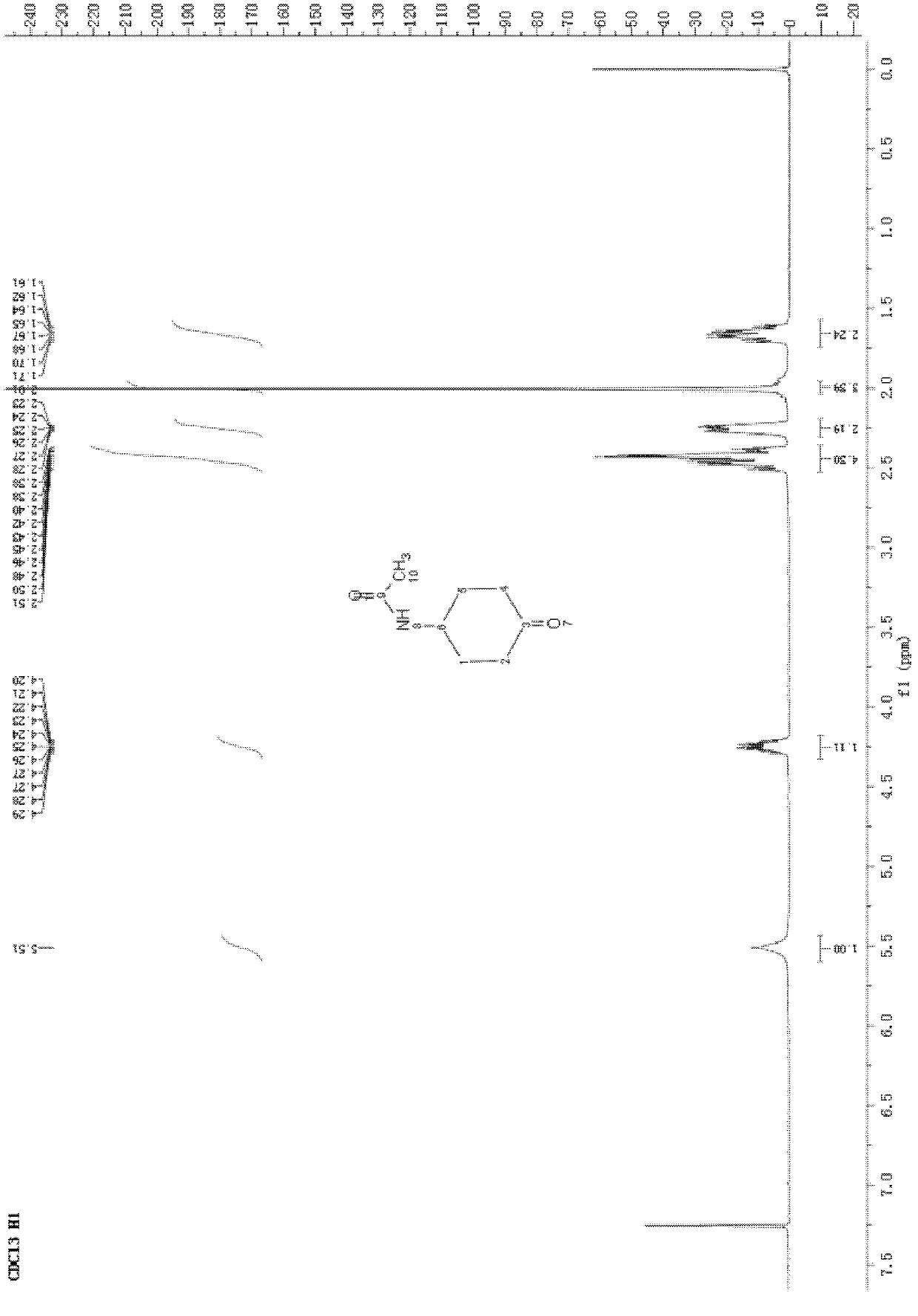
[0031] Under mechanical stirring in a cooling bath at -3°C, TEMPO (0.79 g, 5 mmol), sodium bromide (0.52 g, 5 mmol) were added to 60 ml of acetone, and 4-acetamidocyclohexanol (or the hydrogenated product of acetaminophen) was obtained. ) (15.7g, 100mmol), stir for half an hour, dropwise at a concentration of 10% sodium hypochlorite (170g, 230mmol) below 10 ° C, after the reaction, reclaim acetone, steam off 1 / 2 of the water, and extract 3 times with dichloromethane, 60ml each time, the organic layers were combined, washed with water, washed with saturated brine, dried with 4g of anhydrous sodium sulfate, and the solvent was recovered by distillation under reduced pressure to obtain 15.4g of crude product, which was recrystallized from ethyl acetate to obtain a white crystalline solid 11.8 g (melting point 135-136°C).
[0032] 1 H NMR (400MHz, CDCl 3 , δ, ppm): 5.51(s, 1H), 4.25(m, 1H), 2.55-2.34(m, 4H), 2.31-2.20...
Embodiment 2
[0033] Example 2: Preparation of 4-acetamidocyclohexanone
[0034]Under mechanical stirring in a cooling bath at -3°C, TEMPO (0.16 g, 1 mmol), sodium bromide (0.52 g, 5 mmol) were added to 60 ml of acetone, and 4-acetamidocyclohexanol (or the hydrogenation product of acetaminophen) was taken. ) (15.7g, 100mmol), stir for half an hour, dropwise at a concentration of 10% sodium hypochlorite (170g, 230mmol) below 10 ° C, after the reaction, reclaim acetone, steam off 1 / 2 of the water, and extract 3 times with dichloromethane, 60ml each time, the organic layers were combined, washed with water, washed with saturated brine, dried with 4g of anhydrous sodium sulfate, and the solvent was recovered by distillation under reduced pressure to obtain 9.6g of crude product, which was recrystallized from ethyl acetate to obtain a white crystalline solid 7.7g.
Embodiment 3
[0035] Example 3: Preparation of 4-acetamidocyclohexanone
[0036] Under mechanical stirring in a cooling bath at -3°C, TEMPO (0.79 g, 5 mmol), sodium bromide (0.52 g, 5 mmol) were added to 60 ml of acetone, and 4-acetamidocyclohexanol (or the hydrogenated product of acetaminophen) was obtained. ) (15.7g, 100mmol), stir for half an hour, dropwise at a concentration of 5% sodium hypochlorite (340g, 230mmol) below 10 ° C, after the reaction, reclaim acetone, steam off 1 / 2 of the water, extract 3 times with dichloromethane, 60ml each time, the organic layers were combined, washed with water, washed with saturated brine, dried with 4g of anhydrous sodium sulfate, and the solvent was recovered by distillation under reduced pressure to obtain 2.1g of an oily product, which could not be recrystallized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com