Method for preparing dinitrogen heterocyclooctatetraene
A cyclooctatetraene and diazepine technology, which is applied in the field of high-purity, diazacyclooctatetraene preparation, and high-efficiency preparation of diazacyclooctatetraene, can solve the problem of harsh preparation conditions, easy polymerization, and product Expensive and other problems, to simplify the difficulty of synthesis conditions, reduce one-step reaction, and reduce production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
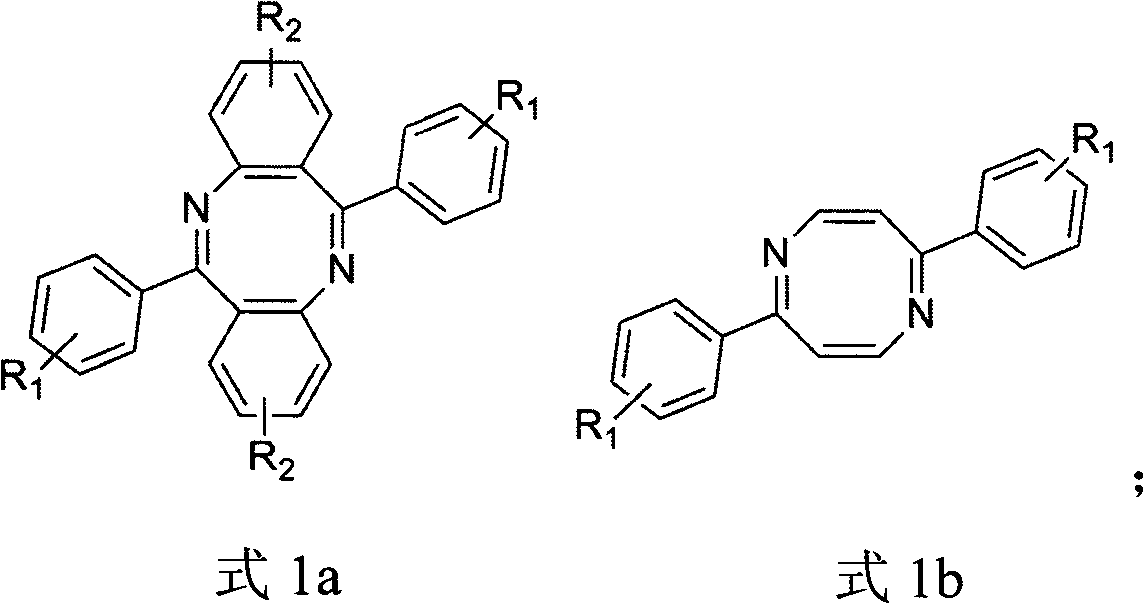
[0018] The method that the present invention proposes to prepare diazacyclooctene is as follows:
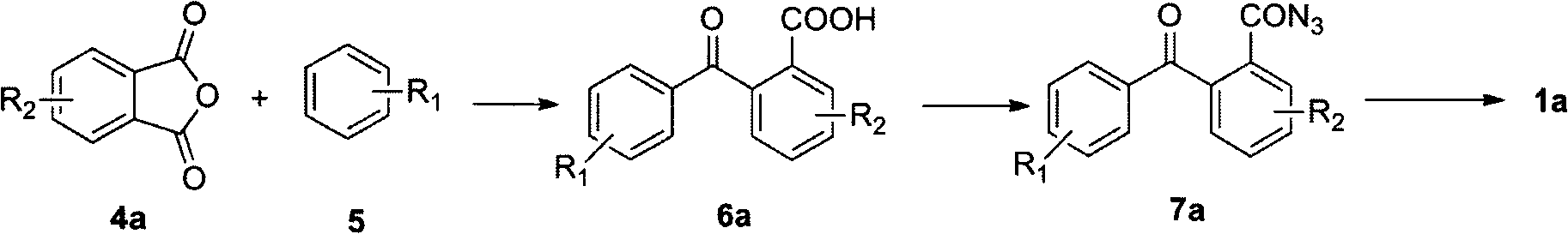
[0019] 1) Preparation of substituted benzoylbenzoic acid: using anhydrous aluminum chloride as Friedel-Crafts reagent, anhydride reacts with aromatic compound to replace benzoylbenzoic acid;
[0020] 2) Preparation of benzoyl azide: first, benzoyl chloride is prepared by using substituted benzoyl benzoic acid under the condition of chlorinating reagent; then the corresponding acyl azide is prepared from benzoyl chloride and azide salt.
[0021] 3) Preparation of diazaoctene: the acyl azide compound is subjected to a ring closure reaction at a certain temperature under the condition of an acidic reagent to prepare diazaoctatetraene.
Embodiment 1
[0023] In a 500mL three-necked round-bottomed flask equipped with a thermometer, a stirrer, and a nitrogen inlet tube, add 200 ml of benzene (5) (R 1 =H) and 50 grams of phthalic anhydride (4a) (R 2 =H), 118 grams of aluminum trichloride, stirred at room temperature for 2 hours, then raised the temperature to 50 degrees Celsius, and reacted with stirring for 5 hours. The reaction was terminated, cooled to room temperature, and poured into a large amount of ice water. Extract with dichloromethane, concentrate the solvent under reduced pressure, and the resulting product is recrystallized with ethanol to obtain o-benzoylbenzoic acid (6a) (R 1 , R 2 =H), yield 85%.
[0024] Phthaloylbenzoic acid (6a) (R 1 , R 2 = H) 3.0 g, 20 ml of thionyl chloride, reflux for 4 hours, concentrate under reduced pressure to prepare o-benzoylbenzoyl chloride; add 30 ml of anhydrous tetrahydrofuran to the system, stir to dissolve and put it in an ice bath; configure Add 5 ml of an aqueous solu...
Embodiment 2
[0035] With embodiment 1, used benzene compound is bromobenzene, 6,12-bis (4'-bromophenyl) dibenzo (b, f) diazacyclooctatetraene (1a) (R 1 =Br,R 2 =H), the overall yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com