Ultrasonic preparation method for imatinib mesylate crystal
A technology of imatinib mesylate and imatinib base, which is applied in the field of ultrasonic preparation of imatinib mesylate crystals, and can solve the problem of poor product stability, deterioration of imatinib, and methanesulfonic acid. Excessive and other problems, to achieve the effect of low solvent residue, firm binding state and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
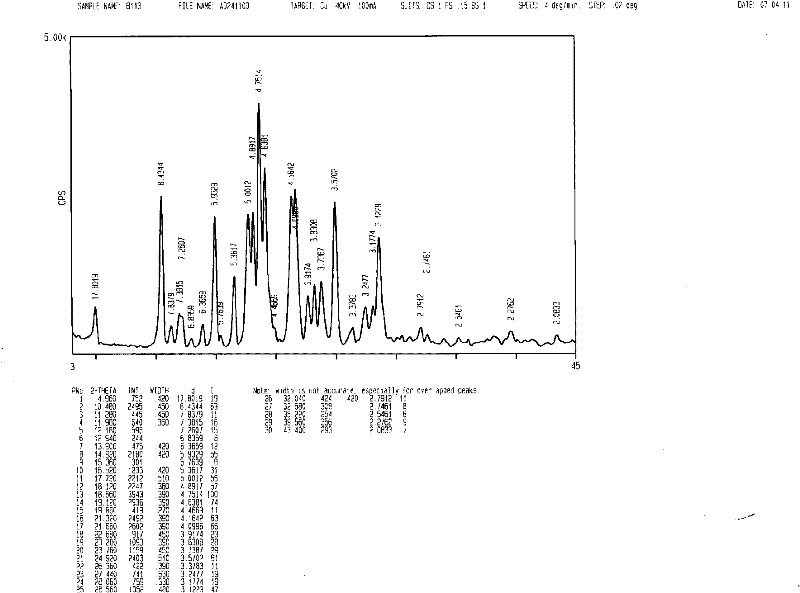
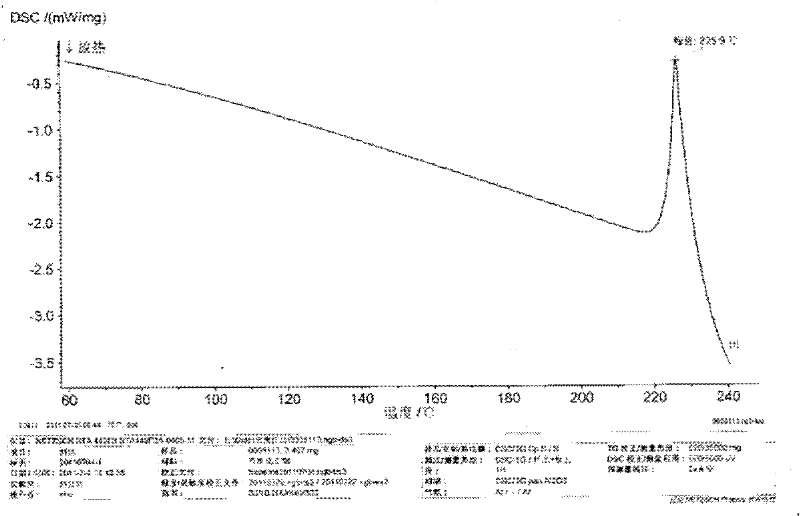
[0023] In a 2000mL reaction flask, add 50.0g of imatinib base and 1200ml of acetone, turn on the ultrasonic equipment, the ultrasonic condition is 24KHz, the temperature of the water bath is raised to 50°C, add 4.96g of methanesulfonic acid in 50ml of acetone solution dropwise in 2 hours, drop After the addition, slowly cool down to 0-5°C, filter, wash the filter cake with acetone, and dry under vacuum (-0.095MPa) at 50°C to obtain 57.1g of imatinib mesylate white crystals with a purity of 99.9% and a yield of 95.6 %. The XRD patterns and DSC patterns of the prepared crystals are shown in figure 1 and figure 2 , conforming to the α-crystal form.
Embodiment 2
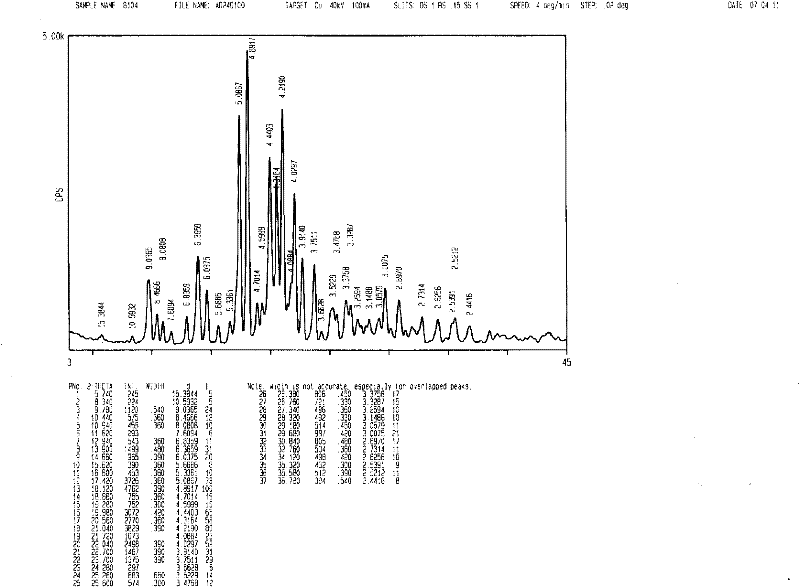
[0025] In a 1000mL reaction bottle, add 50.0g imatinib base and 200ml butanone, turn on the ultrasonic equipment, the ultrasonic condition is 23KHz, heat the water bath to 60°C, add 4.96g methanesulfonic acid solution in 50ml butanone dropwise for 2 hours , after the dropwise addition was completed, the temperature was slowly lowered to 0-5° C., filtered, the filter cake was washed with butanone, and dried under vacuum (-0.095 MPa) at 50° C. to obtain 56.6 g of imatinib mesylate white crystals with a purity of 99.9%. The yield is 94.8%, conforming to the α-crystal form.
Embodiment 3
[0027] In a 2000mL reaction bottle, add 50.0g imatinib base and 1200ml methyl isoamyl ketone, turn on the ultrasonic equipment, the ultrasonic condition is 21KHz, raise the temperature of the water bath to 70°C, add 50ml of 4.96g methanesulfonic acid dropwise for 1 hour Methyl isoamyl ketone solution, after the dropwise addition, slowly cool down to 0-5°C, filter, wash the filter cake with methyl isoamyl ketone, and dry under vacuum (-0.095MPa) at 50°C to obtain 57.9g of ima mesylate Tini is a white crystal with a purity of 99.9% and a yield of 97.0%, conforming to the α-crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com