Non-aqueous electrolyte for lithium iron phosphate battery
A lithium iron phosphate battery, non-aqueous electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as poor compatibility at low temperature performance, and achieve improved electrical conductivity, improved stability, and improved time life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
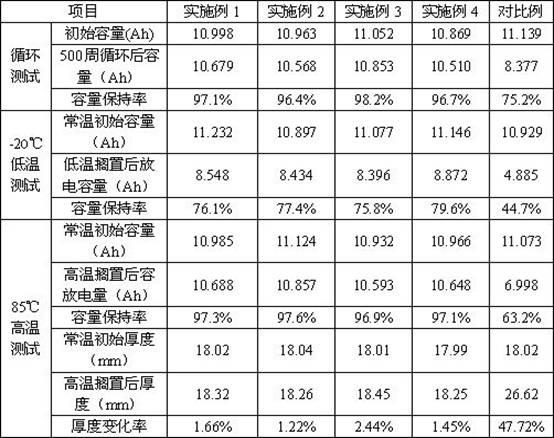
Embodiment 1
[0040] Preparation of non-aqueous electrolyte: at room temperature, in a glove box with dry air (moisture <20PPM), accurately weigh 26.98 grams of ethylene carbonate, 26.98 grams of dimethyl carbonate, and 26.98 grams of ethyl methyl carbonate with an electronic balance , 1.5 grams of vinylene carbonate, 2 grams of 1,3-propane sultone, 3 grams of fluoroethylene carbonate, 0.01 grams of hexamethyldisilazane, 0.05 grams of trimethyl phosphite and 12.50 grams of lithium hexafluorophosphate; Then, the above-mentioned various raw materials were added into a Erlenmeyer flask with a ground mouth, and stirred until the lithium hexafluorophosphate was completely dissolved, and the organic solvents were evenly mixed to obtain 100 grams of non-aqueous electrolyte.
Embodiment 2
[0042] The preparation method of this example is the same as Example 1, except that the raw materials used are 27.12 grams of ethylene carbonate, 27.12 grams of dimethyl carbonate, 27.12 grams of diethyl carbonate, 2.00 grams of vinyl vinylene carbonate, 1,4-butane 2.00 grams of sultone, 2.00 grams of fluoroethylene carbonate, 0.02 grams of heptamethyldisilazane, 0.12 grams of trimethyl phosphite and 12.50 grams of lithium hexafluorophosphate.
Embodiment 3
[0044] The preparation method of this embodiment is the same as that of Example 1, except that the raw materials used are 24.40 grams of ethylene carbonate, 24.40 grams of dimethyl carbonate, 24.40 grams of ethyl methyl carbonate, 8.15 grams of propylene carbonate, 1.00 grams of vinyl vinylene carbonate, 2.00 g of 1,3-propane sultone, 2.00 g of bisfluoroethylene carbonate, 0.01 g of heptamethyldisilazane, 0.14 g of triphenyl phosphite and 13.50 g of lithium hexafluorophosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com