Cefuroxime axetil granules and preparation method
A technology of cefuroxime axetil and granules, which is applied in the direction of pharmaceutical formulas, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor dispersion and residual bitterness of preparations, and achieve the promotion of drug dissolution, Improve the dissolution rate and mask the effect of bitter taste
Active Publication Date: 2012-07-25
SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
View PDF2 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the granules obtained by the above method are poorly dispersed in water during the preparation process due to the presence of stearic acid, and the preparation still has a bitter taste, causing problems when oral administration to humans, especially infants
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
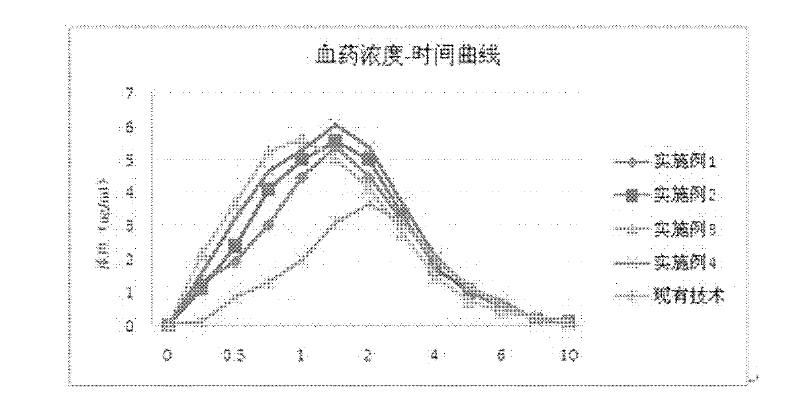
Embodiment 1
Embodiment 2
Embodiment 3
[0055] The preparation method of a cephalosporin granules includes the following steps (the amount of each component is described in Table 3):
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more PUM
 Login to view more
Login to view more Abstract
The invention discloses cefuroxime axetil granules, which comprise the following compositions by weight percent: 5 to 40 percent of cefuroxime axetil, 5 to 60 percent of polymer, 10 to 80 percent of fillers, 3 to 20 percent of disintegrant, 0.01 to 5 percent of sweetening agents, 0.05 to 5 percent of bond and 0.05 to 5 percent of flavoring, wherein the polymer is one or more of polyethyleneglycol-polyvinyl acetate-vinyl caprolactam copolymer, vinylpyrrolidone-vinyl acetate copolymer, hydroxy propyl cellulose, polymethylmethacrylate and gastric-soluble acrylic resin. The invention also discloses a preparation method of the cefuroxime axetil granules. The prepared cefuroxime axetil granules can well cover the bitterness of the medicine, and higher cefuroxime biological availability and higher quality stability can be realized.
Description
Technical field [0001] The invention involves the field of drug preparation technology, which specifically involves a cephalosporin spinth granules and their preparation methods. Background technique [0002] Cefachebal ester is oral cephalosporin antibiotics. It has a wide range of antibacterial spectrum and strong antibacterial activity. It has good antibacterial activity for Gram-positive microorganisms and Gram-negative microorganisms.Primary and other advantages.However, the raw materials of cephalosporidine are unstable to wet and heat, poor water solution, water gathering group, and the taste is extremely bitter, which is not conducive to children's administration. [0003] Glaxosmithkline (GSK) South Korean patent application No.1995-0009097 revealed a method of preparing the particles to cover the bitterness of cephalosporin, including the following steps: scattered the drugs in the melted stereotic acid, the spray drying income dispersed, and the spray was dispersed.And...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more Application Information
Patent Timeline
 Login to view more
Login to view more IPC IPC(8): A61K9/16A61K31/546A61K47/32A61K47/34A61K47/36A61K47/38A61P31/04
CPCA61K9/1652A61K47/38A61K9/16A61K31/546A61K47/34A61K47/32A61K9/1635A61K9/0095A61P31/04
Inventor 闫志刚邓宝军黄艳曾环想王泳罗炳锋李娟权超
Owner SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap