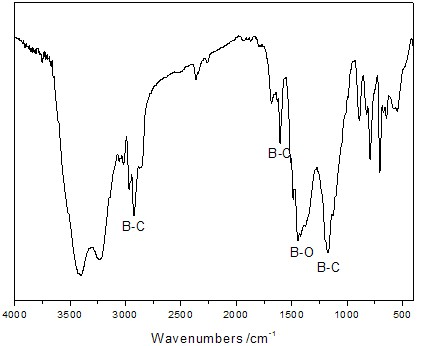
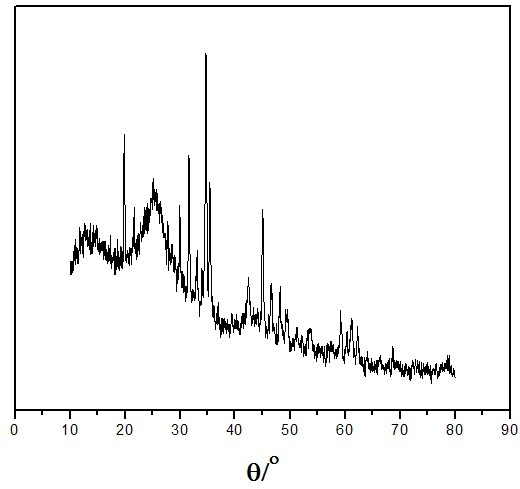
Preparation method for boron carbide precursor
A technology of precursor and boron carbide, which is applied in the field of preparation of boron carbide precursor, can solve the problems of low yield, high production cost, and poor solubility of boron carbide precursor, and achieve low price, low manufacturing cost and good solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This embodiment includes the following steps:
[0022] (1) Take 40ml of borane complex and 20g of 1,5-hexadiene for later use;
[0023] (2) Add the 1,5-hexadiene described in step (1) into a 250ml three-necked flask with a stirring and distillation device; then vacuumize the three-necked flask and fill it with dry nitrogen until the pressure gauge returns to zero, repeat three times, To get rid of the air and moisture in it, and then pre-cool the reactor to -10°C;
[0024] (3) In the reactor described in step (2), add the borane described in step (1); 2 Under the protection of the atmosphere, the reaction was continuously stirred at room temperature, the stirring rate was 240r / min, and the reaction was carried out for 7h;
[0025] (4) in N 2 Under the protection of the atmosphere, the temperature of the system was raised to 260°C at a heating rate of 2°C / min, and the solvent component in the system was distilled off under reduced pressure; cooled to room temperature,...
Embodiment 2
[0028] (1) Take 40ml of pentaborane and 20g of 1-octene for later use;
[0029] (2) In a 250ml three-necked flask with a stirring and distillation device, add 1-octene described in step (1); then vacuumize the three-necked flask, fill it with dry nitrogen until the pressure gauge returns to zero, and repeat it three times to eliminate the air and moisture, and then pre-cool the reactor to -5°C;
[0030] (3) In the reactor described in step (2), add the pentaborane described in step (1); 2 Under the protection of the atmosphere, the reaction was continuously stirred at room temperature, the stirring rate was 200r / min, and the reaction was carried out for 10h;
[0031] (4) in N 2 Under the protection of the atmosphere, the temperature of the system was raised to 240°C at a heating rate of 3°C / min, and the solvent in the system was removed by distillation under reduced pressure; cooled to room temperature, it was ready.
[0032] After analysis, the obtained polymer mainly cont...
Embodiment 3
[0034] (1) Take 60ml of borane complex, 20g of 1,5-hexadiene, 10g of 1-octene, set aside;
[0035] (2) Add 1-octene and 1,5-hexadiene described in step (1) into a 250ml three-necked flask with a stirring and distillation device; then vacuumize the three-necked flask and fill it with dry nitrogen until the pressure gauge returns Zero, repeat three times to get rid of the air and moisture, and then pre-cool the reactor to -15°C;
[0036] (3) In the reactor described in step (2), add the borane complex described in step (1); 2 Under the protection of the atmosphere, the reaction was continuously stirred at room temperature, the stirring rate was 180r / min, and the reaction was carried out for 11h;
[0037] (4) in N 2 Under the protection of the atmosphere, the temperature of the system was raised to 250°C at a heating rate of 2°C / min, and the solvent components in the system were removed by distillation under reduced pressure; cooled to room temperature, and the product was read...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com