Usage of human interferon in preparation of medicines for treatment or prevention of HPV (human paillomavirus) related diseases
A technology of interferon and interferon alpha, which is applied in the field of medicine to achieve the effect of enhancing the body's antiviral ability, inhibiting the virus, and having good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
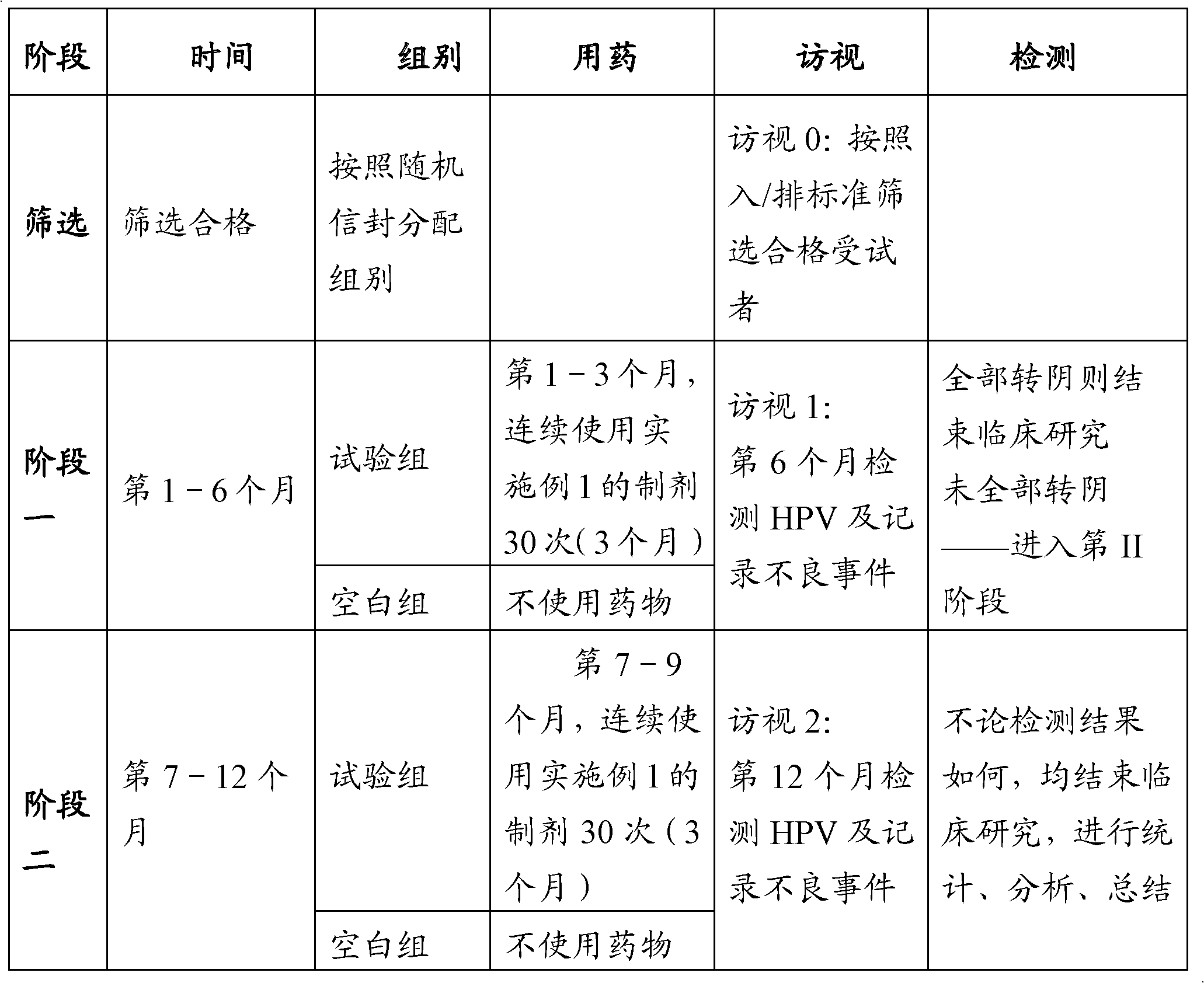
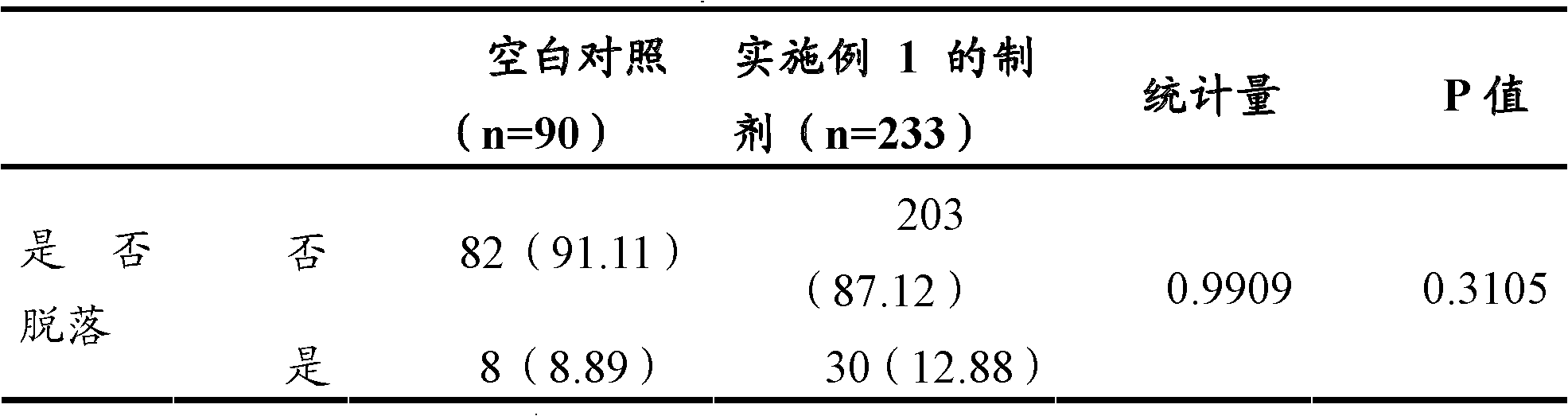
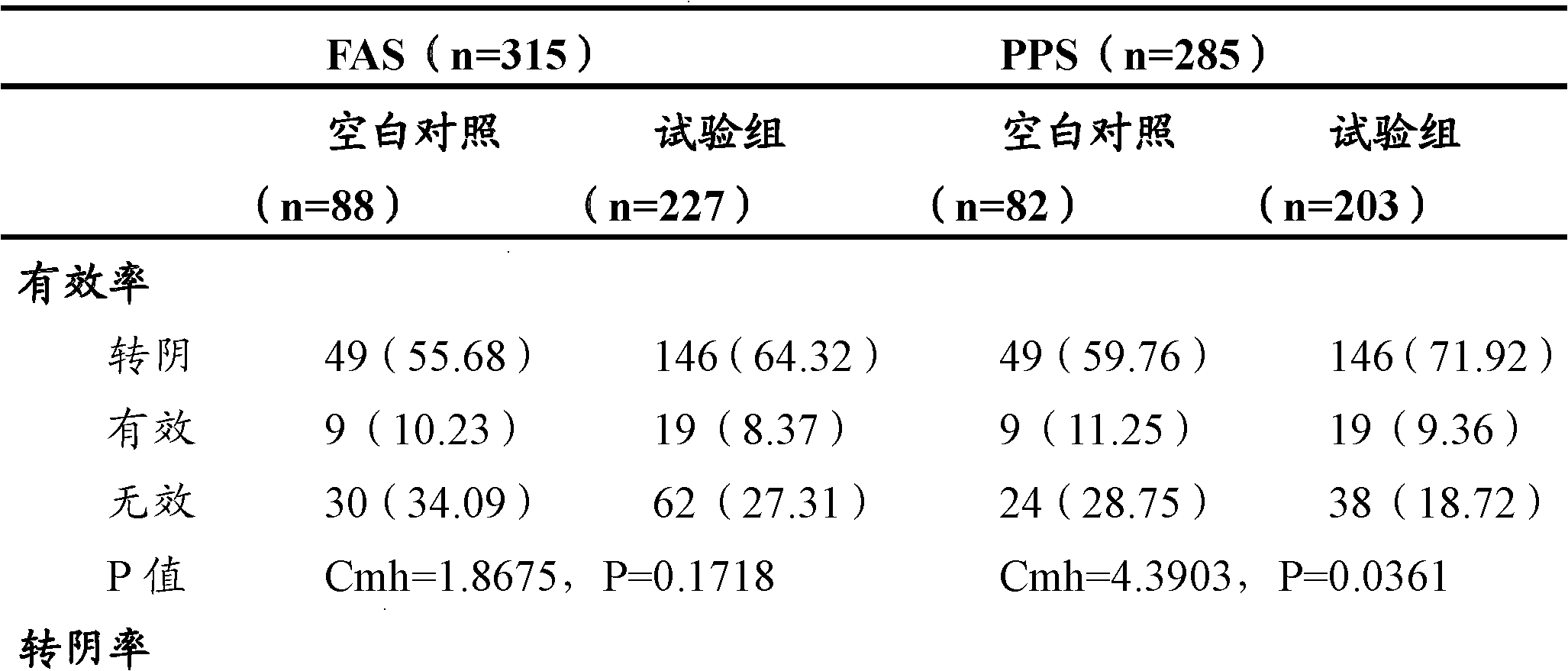
Examples
Embodiment 1
[0026] Embodiment 1, preparation prescription technology:
[0027] (1) Treatment of human interferon stock solution:
[0028] Add human serum albumin according to the volume of human interferon stock solution, so that the final concentration of human serum albumin is 5% (W / V), then add an equal volume of stabilizer SDS according to the total volume of the human interferon solution that has added albumin, Mix evenly, heat in a water bath for 0.5-3min, cool, divide and freeze-dry to powder.
[0029] (2) Matrix preparation:
[0030] Accurately weigh 200g of sodium carboxymethylcellulose, 500ml of glycerin, and 20g of sorbic acid, mix them, add water for injection to 10,000g, heat to 100°C and mix well, and when the matrix is cooled to 40°C, adjust the pH to 7.5 and set aside.
[0031] (3) Preparation of human interferon preparation:
[0032]Add human interferon powder in the matrix (add human interferon powder 1.0 × 10 in every 1000g matrix 9 SI units), stirring constantly ...
Embodiment 2
[0033] Embodiment 2, preparation prescription process:
[0034] (1) Treatment of human interferon stock solution:
[0035] Add human serum albumin according to the volume of human interferon stock solution, so that the final concentration of human serum albumin is 1% (W / V), and then add an equal volume of stabilizer reduced form according to the total volume of the human interferon solution that has been added with albumin Glutathione, mixed evenly, heated in a water bath for 0.5 min, cooled, subpackaged and freeze-dried into powder.
[0036] (2) Matrix preparation:
[0037] Accurately weigh 50g of sodium carboxymethylcellulose, 1500ml of glycerin, and 10g of sorbic acid, mix them, add water for injection to 10,000g, heat to 60°C and mix well, and when the matrix is cooled to 10°C, adjust the pH to 4.0 and set aside.
[0038] (3) Preparation of human interferon preparation:
[0039] Add human interferon powder in the matrix (add human interferon powder 1.0 × 10 in every 1...
Embodiment 3
[0040] Embodiment 3 preparation prescription technology:
[0041] (1) Treatment of human interferon stock solution:
[0042] Add human serum albumin according to the volume of the human interferon stock solution, so that the final concentration of human serum albumin is 3% (W / V), and then add an equal volume of stabilizer vitamin C according to the total volume of the human interferon solution that has been added with albumin , mixed evenly, heated in a water bath for 2 minutes, cooled, subpackaged and freeze-dried into powder.
[0043] (2) Matrix preparation:
[0044] Accurately weigh 100g of sodium carboxymethylcellulose, 750ml of glycerin, and 15g of sorbic acid, mix them, add water for injection to 10,000g, heat to 80°C and mix well, and when the matrix is cooled to 30°C, adjust the pH to 9.5 and set aside.
[0045] (3) Preparation of human interferon preparation:
[0046] Add human interferon powder in the matrix (add human interferon powder 1.0 × 10 in every 1000g m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com