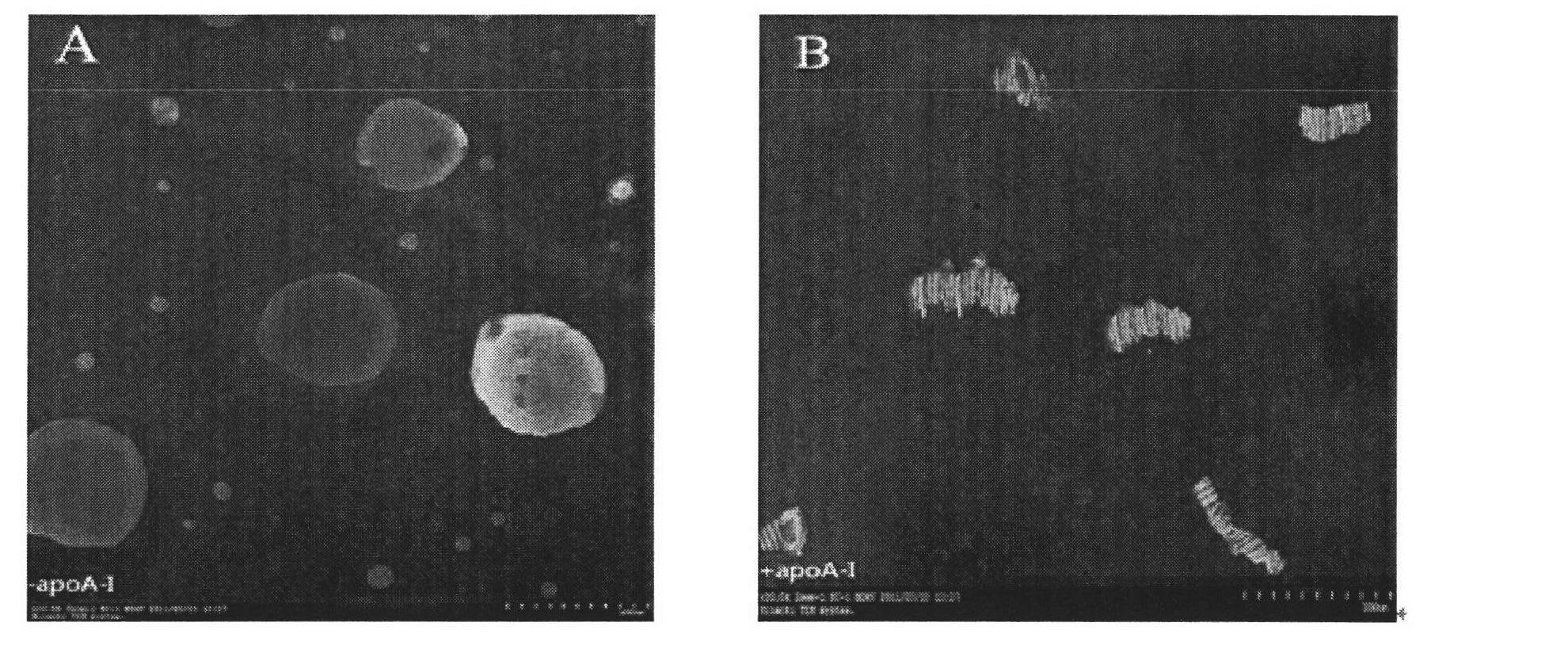
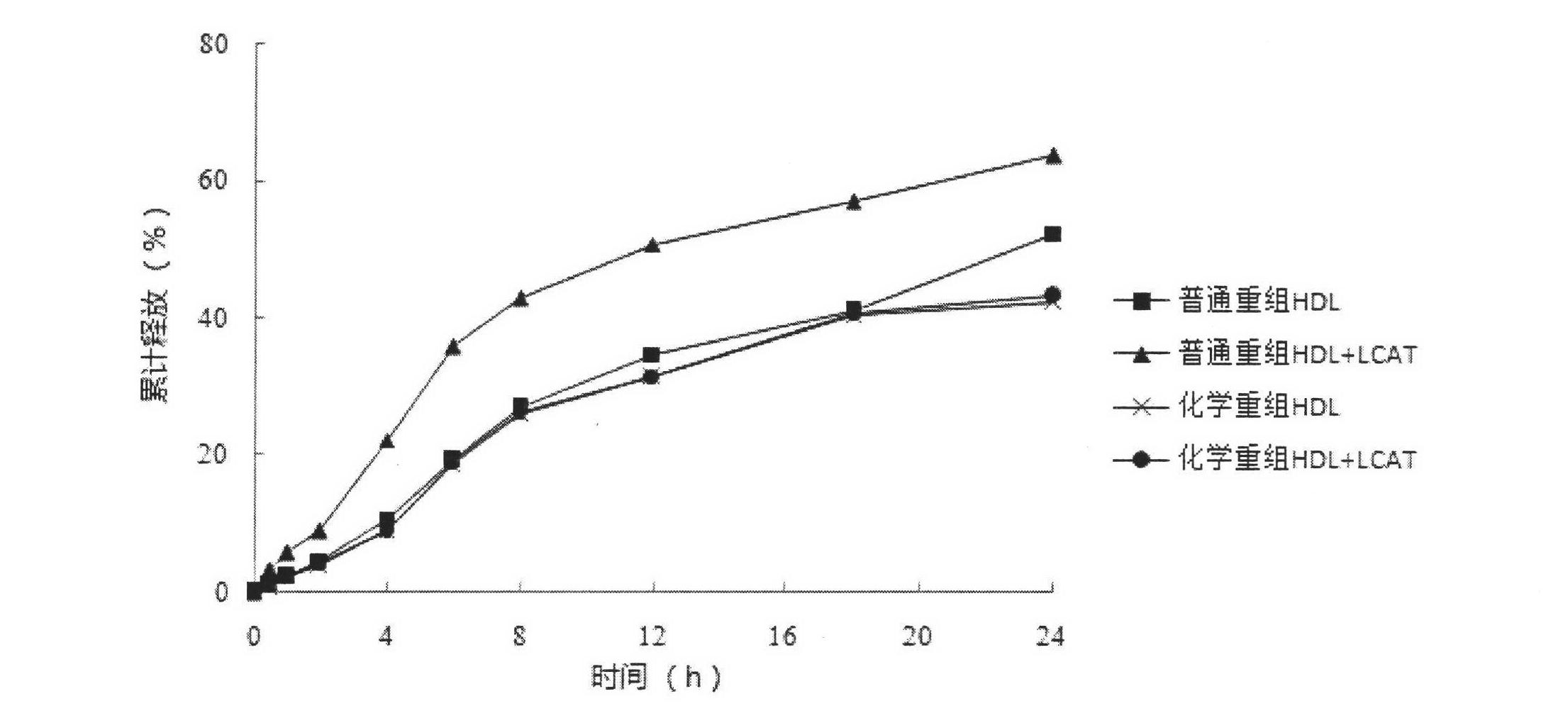
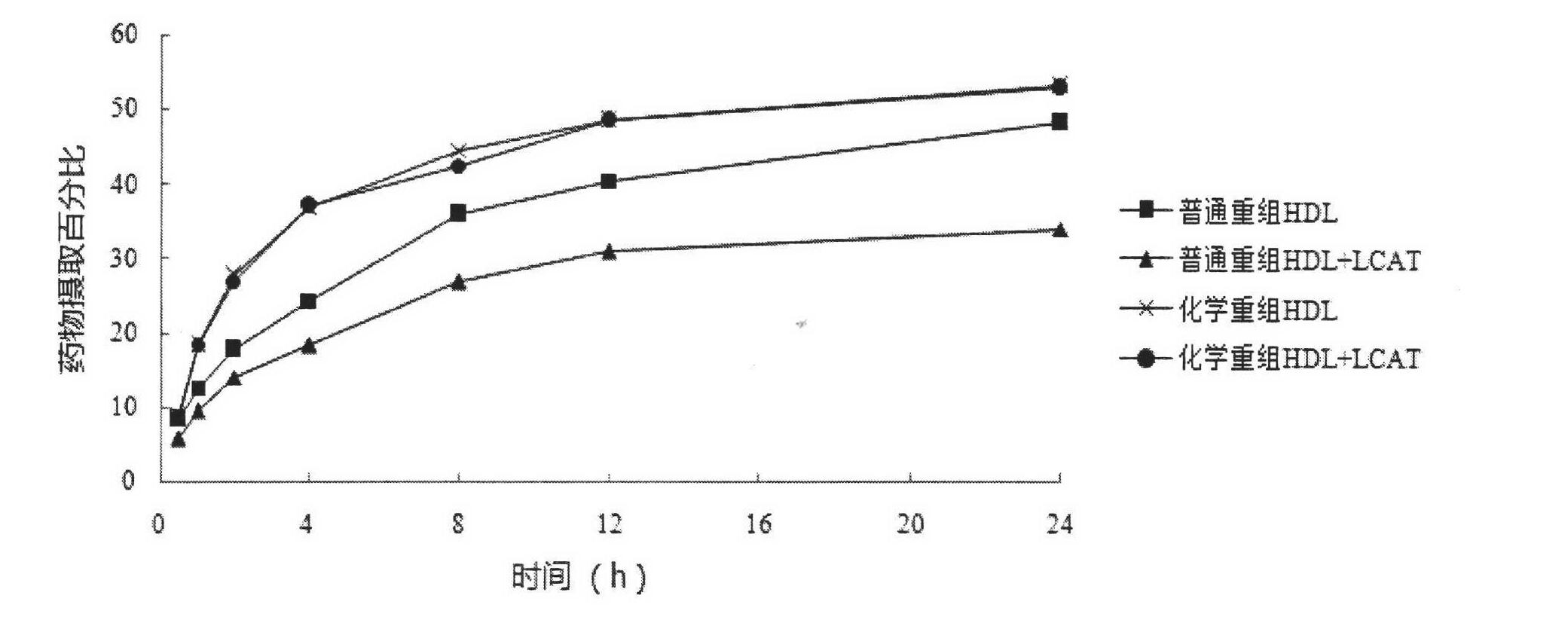
Novel chemical restructuring high-density lipoprotein drug carrying system with targeted and double anti-tumor effects and application
A high-density lipoprotein and drug-carrying system technology, applied in the field of recombinant high-density lipoprotein carriers, can solve the problems of recombinant HDL that have not been reported and the physical stability of rHDL reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The composition of the prescription is as follows: Paclitaxel: 0.01g
[0021] Egg phospholipids: 0.2g
[0022] Modified Cholesterol: 0.02g
[0023] Sodium cholate: 0.02g
[0024] apoA-I: 0.2g
[0025] Organic Phase: Ethanol
[0026] Hydration medium: PBS buffer pH 7.4
[0027] Preparation process: put the prescribed amount of medicine, egg phospholipid, and modified cholesterol in a vial, add an appropriate amount of ethanol, dissolve it by ultrasonication, transfer it into an eggplant-shaped bottle, place it in a rotary evaporator, remove the organic phase under reduced pressure, and vacuum Dry, add an appropriate amount of hydration medium dissolved with sodium cholate, ultrasonically disperse evenly into a translucent suspension, and then use a probe to ultrasonically pulverize to make it a transparent and opalescent liquid, pass through a 0.22 μm filter membrane, add apolipoprotein, After dissolving, place in a refrigerator at 4°C, stir magnetically at 600 rpm ...
Embodiment 2
[0029] The composition of the prescription is as follows: Paclitaxel: 0.01g
[0030] Egg phospholipids: 0.2g
[0031] Modified cholesterol 0.02g
[0032] Sodium cholate: 0.01g
[0033] Sodium lauryl sulfate: 0.01g
[0034] apoA-I: 0.2g
[0035] Organic Phase: Ethanol
[0036] Hydration medium: PBS buffer pH 7.4
[0037] The preparation process is the same as in Example 1. The average particle size of the prepared rHDL is 55nm, the encapsulation efficiency is 85.07%, and the protein binding rate is 45.9%.
Embodiment 3
[0039] The composition of the prescription is as follows: Paclitaxel: 0.01g
[0040] Egg phospholipids: 0.2g
[0041] Sodium cholate: 0.02g
[0042] Cholesterol modified apoA-I: 0.3g
[0043] Organic Phase: Ethanol
[0044] Hydration medium: PBS buffer pH 7.4
[0045] Preparation process: put the prescribed amount of medicine and egg phospholipids in a vial, add an appropriate amount of ethanol, dissolve it with ultrasound, transfer it into an eggplant-shaped bottle, place it in a rotary evaporator, remove the organic phase under reduced pressure, dry it in vacuum, add Appropriate amount of hydration medium dissolved with sodium cholate, ultrasonically dispersed into a translucent suspension, and then ultrasonically crushed with a probe to make it a transparent and opalescent liquid, passed through a 0.22 μm filter membrane, and dissolved by adding cholesterol-modified apolipoprotein Afterwards, place in a refrigerator at 4°C, stir magnetically at 600 rpm for 5 hours, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com