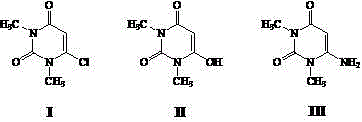
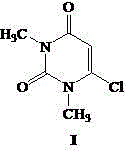
Process for preparing 6-chlorine-1,3-dimethyl uracil
A technology of dimethyluracil and hydroxyl, which is applied in the field of preparation of 6-chloro-1,3-dimethyluracil I, can solve the problems of low product quality, high production cost, difficult operation, etc., and achieve the product The effect of high yield, low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Add 150 kg of 6-amino-1,3-dimethyluracil, 600 kg of water, and 50 kg of concentrated sulfuric acid (98%) into a 1000 L reactor, gradually heat up to reflux, keep for 5 hours, and cool Crystallized, centrifuged, and dried to obtain 140.5 kg of milky white crystals of 6-hydroxy-1,3-dimethyluracil, with a melting point of 121°C-124°C and a yield of 93.0%. Into a 500 L dry reaction kettle, pump 450 kg of phosphorus oxychloride and 150 kg of phosphorus trichloride respectively, lower the temperature to below 20°C, slowly add 14 kg of water dropwise, and add a total of 50 kg of the above-prepared 6 -Hydroxy-1,3-dimethyluracil, after adding, gradually raise the temperature to reflux, stop heating after 3 hours of heat preservation, slowly inject vacuum, start decompression recovery of phosphorus oxychloride, until about 90℃, the material in the kettle becomes Stop the distillation when it is cloudy, transfer the material to a kettle with 500kg of ice water in advance...
Embodiment 2
[0020] Example 2 Add 150 kg of 6-amino-1,3-dimethyluracil, 400 kg of water, and 150 kg of concentrated hydrochloric acid (36%) into a 1000 L reactor, gradually heat up to reflux, keep for 4 hours, and cool Crystallize below 30°C, centrifuge, freeze the mother liquor below 10°C, precipitate solids, centrifuge, and dry to obtain 139 kg of off-white crystalline product 6-hydroxy-1,3-dimethyluracil, melting point 121°C-124°C, yield The rate is 92.0%. Into a 500 L dry reaction kettle, pump 600 kg of phosphorus oxychloride, lower the temperature to below 20°C, slowly add 20 kg of methanol dropwise, and add a total of 50 kg of 6-hydroxyl-1,3- After the addition of dimethyl uracil, the temperature is gradually raised to reflux. After 5 hours of heat preservation, the heating is stopped, and the vacuum is slowly injected, and the phosphorus oxychloride is recovered under reduced pressure. When the material in the kettle becomes cloudy at about 90°C, the distillation is stopped. Transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com