Application of poplar glycosyl transferase PtGT2 to catalytic synthesis of phenylpropanoid glucosides
A technology of glycosyltransferase and phenylpropanoid glycoside, applied in directions such as transferase, fermentation, etc., can solve problems such as reports that have not yet been found to be applied, and achieve the effects of low efficiency and huge economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
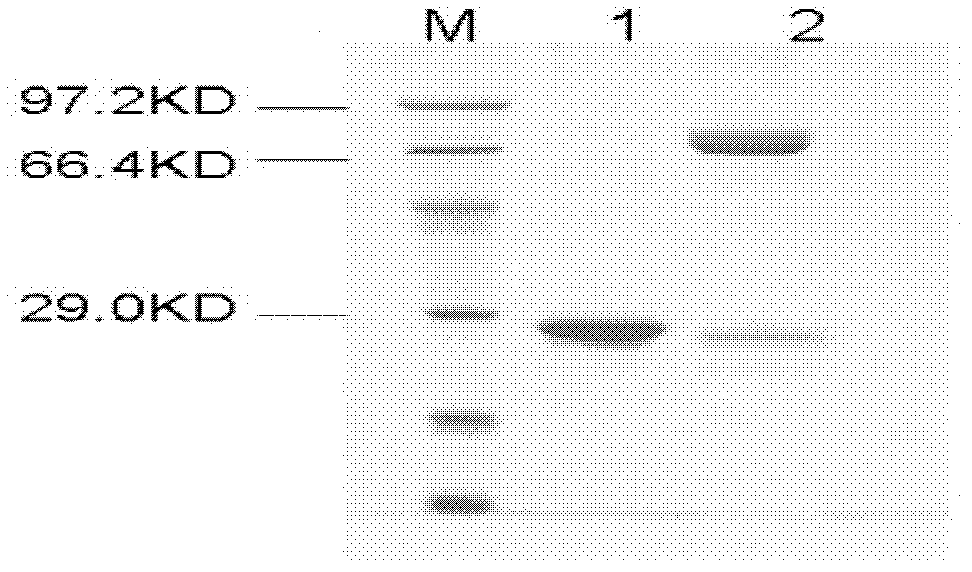
[0025] Example 1 Cloning, prokaryotic expression and enzyme protein purification of poplar glycosyltransferase gene PtGT2
[0026] 1. Cloning of poplar glycosyltransferase gene PtGT2
[0027] The glycosyltransferase gene PtGT2 involved in the present invention is cloned from poplar through RT-PCR amplification technology. Firstly, RNA was extracted from young leaves of Populus tomentosa by TRIzol method, and then the coding region cDNA of the gene was amplified by RT-PCR method. A pair of primers used for amplification are: GT2-a: 5'-ATAGGATCCATGCAAAAC ACAAAACCTCA-3'; GT2-b: 5'-ATAGAGCTC CTATGCACCTTGAGCCTTTGC-3'. The RT-PCR amplification program is: 94°C (pre-denaturation), 5min; 94°C (denaturation), 10s; 55°C (annealing), 15s; 72°C (extension), 2min; 35cycle; 72°C (final extension), 10min. The amplified product is recovered and purified. The amplified target gene PtGT2 was connected to the EcoRV single-digested intermediate vector pBluescript SK to obtain the vector pBGT2...
Embodiment 2
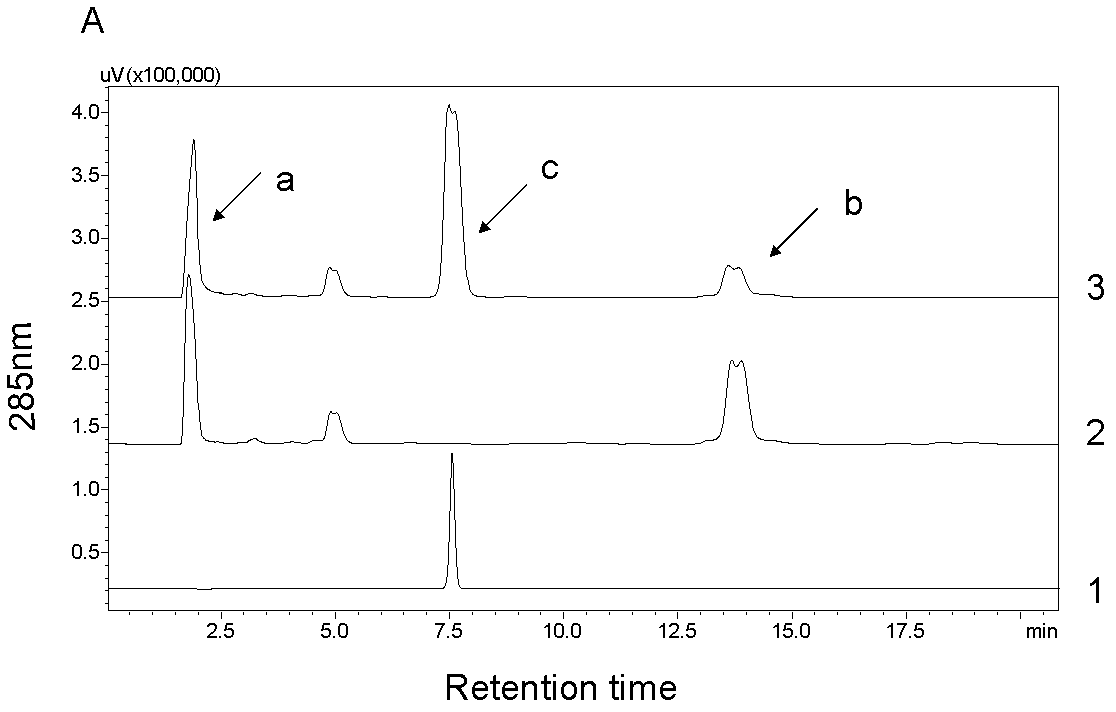
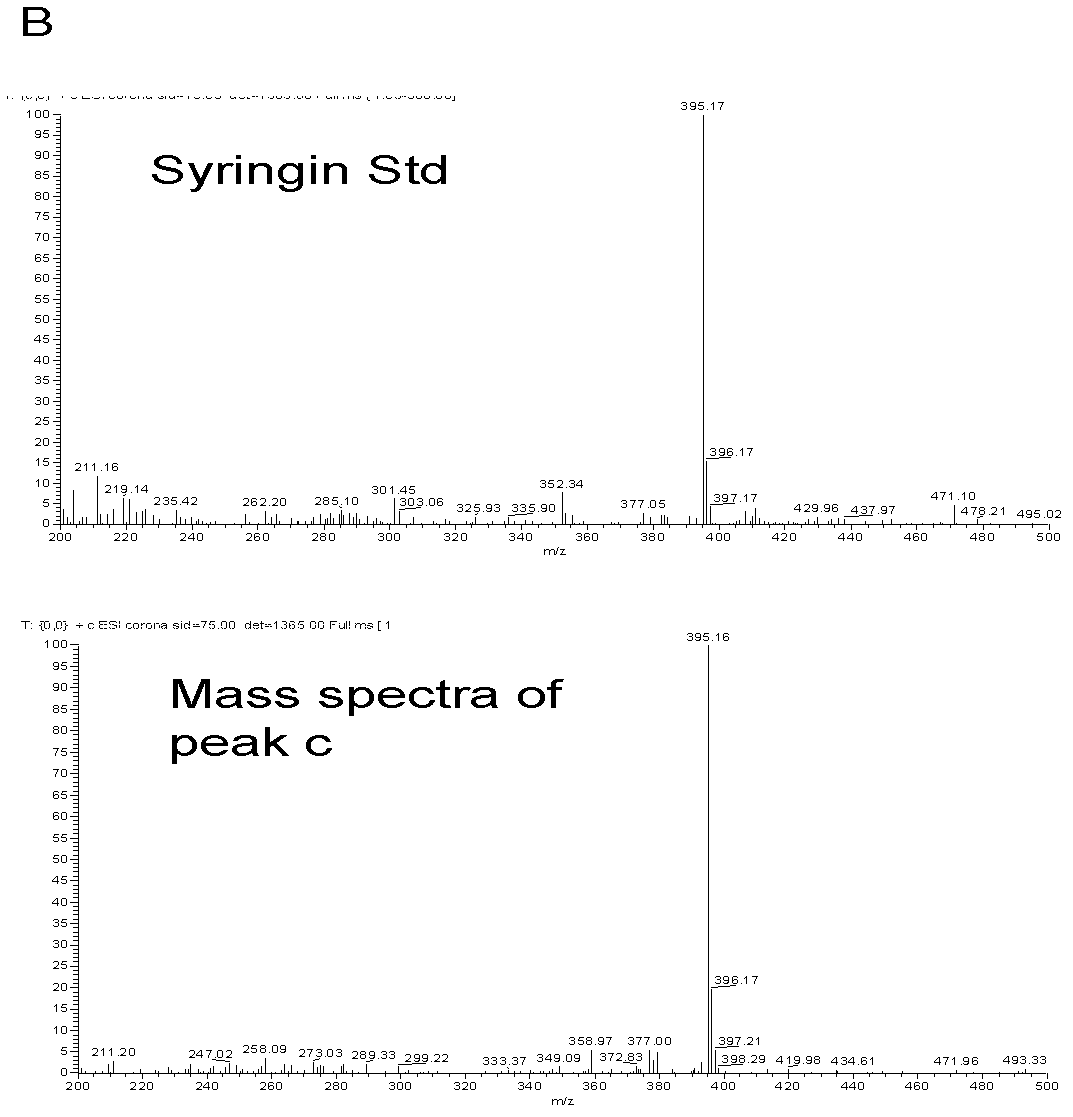
[0036] Example 2 Poplar glycosyltransferase PtGT2 catalyzes the synthesis of phenylpropanoid glycosides
[0037] 1. Enzyme-catalyzed reaction of phenylpropanoid
[0038] The fusion protein of the purified poplar glycosyltransferase PtGT2 (GST-PtGT2, which can reflect the catalytic activity of PtGT2, which is a common practice) is used for in vitro enzyme-catalyzed reaction, and six kinds of phenylpropanoid compounds are selected as substrates, including Coniferyl alcohol, sinapyl alcohol, coniferyl aldehyde, sinapinaldehyde, sinapinic acid, ferulic acid. UDP-glucose was used as the sugar donor in the catalytic reaction. The enzymatic reaction system is as follows:
[0039]
[0040] Put the above mixed reaction system in a constant temperature water bath at 30°C and react for 3 hours. The reaction was then terminated by adding 20 μl of trichloroacetic acid at a concentration of 240 mg / ml. The reaction tubes were snap-frozen with liquid nitrogen and stored at -20°C until ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com