Cupronickel alloy and manufacturing method thereof for coinage
A technology of cupronickel and alloy, which is applied in the field of cupronickel alloy for coinage and its preparation, can solve the problems of unsatisfactory corrosion resistance, spalling, and high nickel content of the alloy, and achieve enhanced silvery white luster, improved corrosion resistance, and improved corrosion resistance. The effect of abrasive properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
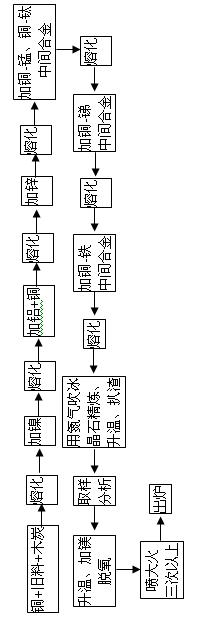
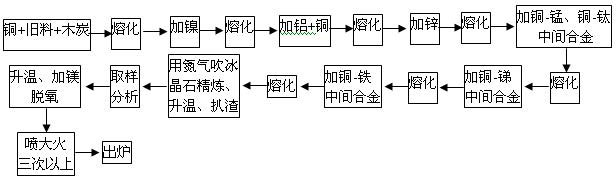
[0041] Put Cu with a mass percentage of 46% into a power frequency furnace for smelting. After the copper starts to soften, cover a layer of charcoal on the copper surface. The amount of charcoal is 15-25kg per ton of copper. The melting temperature is 1180-1210°C. Add 8.3% Ni by mass to the molten copper, so that nickel metal can be dissolved into the copper liquid through alloying. After Ni is melted, 0.5% Al and 15% Cu are added to the molten copper; by adding cold copper and aluminum at the same time, the heat of dissolution of aluminum can be used to melt the cold copper to reduce energy consumption and The purpose of metal burning. After the added Al and Cu are melted, add 17.0% Zn by mass percentage to the copper liquid; adopt the purpose of adding aluminum first and then add zinc, can use low-priced metal aluminum to deoxidize the copper liquid, and use aluminum on the surface of the copper liquid A dense aluminum oxide protective film is formed to reduce the copper...
Embodiment 2
[0048] Put Cu with a mass percentage of 50% into a power frequency furnace for smelting, and after the copper starts to soften, cover a layer of charcoal on the copper surface. The amount of charcoal is 15-25kg per ton of copper. The melting temperature is 1180-1210°C. Add 9.2% Ni by mass percentage to the molten copper liquid, and add 0.7% Al and 14.5% Cu by mass percentage to the copper liquid after Ni is melted. After the added Al and Cu are melted, add 18.0% Zn by mass percentage to the copper liquid; after the metal Zn is melted, add Cu-Mn and Cu-Ti master alloy to the copper liquid to make the Mn and Ti in the finished alloy The mass percent is 4.0% and 2.2%; Add Cu-Sb master alloy in copper liquid, make the mass percentage of Sb in the finished alloy be 0.3%; Add Cu-Fe master alloy in copper liquid, make the mass percentage of Fe in the finished alloy The percentage is 0.7%;
[0049] After the added master alloy is melted, use high-purity nitrogen or argon as carrier...
Embodiment 3
[0052] Put Cu with a mass percentage of 55% into a power frequency furnace for smelting. After the copper starts to soften, cover a layer of charcoal on the copper surface. The amount of charcoal is 15-25kg per ton of copper. The melting temperature is 1180-1210°C. Add 10.2% Ni by mass percentage to the molten copper liquid, and add 0.9% Al and 14.5% Cu by mass percentage to the copper liquid after Ni is melted. After the added Al and Cu are melted, add 20.0% Zn by mass percentage to the copper liquid; after the metal Zn is melted, add Cu-Mn and Cu-Ti master alloy to the copper liquid to make the Mn and Ti in the finished alloy The mass percentages are 5.0% and 2.5%; add Cu-Sb master alloy to the copper liquid to make the mass percentage of Sb in the finished alloy be 0.5%; add Cu-Fe master alloy to the copper liquid to make the mass percentage of Fe in the finished alloy The percentage is 0.9%;
[0053] After the added master alloy is melted, use high-purity nitrogen or ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com