Method for constructing finger-print chromatogram for ginsenosides-containing crude drugs and preparations
A technology of ginsenosides and fingerprints, which is applied to the analysis of materials, material separation, and measuring devices, etc. It can solve problems such as inability to control quality, difficult to achieve baseline separation, and incomplete analysis of characteristic components, so as to achieve accurate quality control and ensure stability. Effects of Sex and Consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
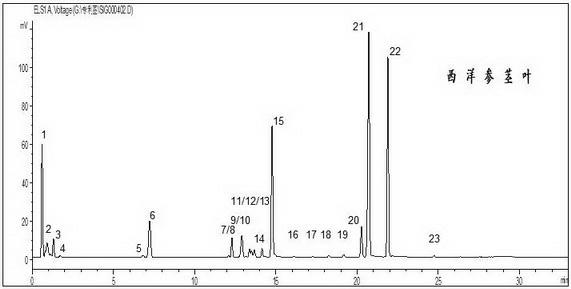
[0045] American ginseng stems and leaves
[0046]1) Preparation of the test solution: Weigh 0.2 g of American ginseng stems and leaves, put it in a Soxhlet extractor, add 100 ml of chloroform, heat and reflux for 1 hour, discard the chloroform solution, evaporate the dregs of the chloroform, add 50 ml of methanol, Heat to reflux for 3 hours, evaporate the extract to dryness at low temperature, add 10 ml of water to dissolve, add petroleum ether (30~60°C) to extract twice, 10 ml each time, discard the ether solution, and pass the water through a D101 macroporous adsorption resin column (1.5 cm inner diameter, 15 cm column length), elute with 70 ml of water, discard the water, then elute with 50 ml of 20% ethanol, discard the 20% ethanol eluate, and then use 80% ethanol 80 ml for elution, collect 70 ml of the eluate, evaporate to dryness, dissolve the residue in methanol, put it in a 10 ml volumetric flask, and constant volume, as the test solution, pass through a 0.22 μm memb...
Embodiment 2
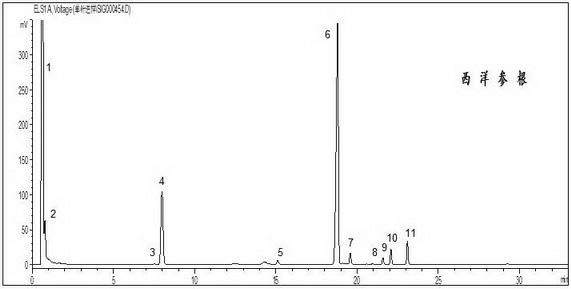
[0083] American ginseng root
[0084] 1) Preparation of the test solution: Weigh 1 g of American ginseng root, put it in a Soxhlet extractor, add 100 ml of methanol, heat and reflux for 3 hours, evaporate the extract to dryness at low temperature, add 50 ml of water to dissolve, add petroleum ether (30 ~60°C) twice, 50 ml each time, discard the ether solution, pass the water solution through a D101 macroporous adsorption resin column (1.5 cm inner diameter, 15 cm column length), elute with 100 ml water, discard water solution, then elute with 100 ml of 20% ethanol, discard the 20% ethanol eluate, continue to elute with 150 ml of 80% ethanol, collect 100 ml of eluate, evaporate to dryness, add methanol to dissolve the residue, and place in 10 ml In a volumetric flask, constant volume was used as the test solution, and passed through a 0.22 μm filter membrane before analysis.
[0085] 2) Preparation of reference solution: accurately weigh ginsenoside Rb 1 , dissolved in meth...
Embodiment 3
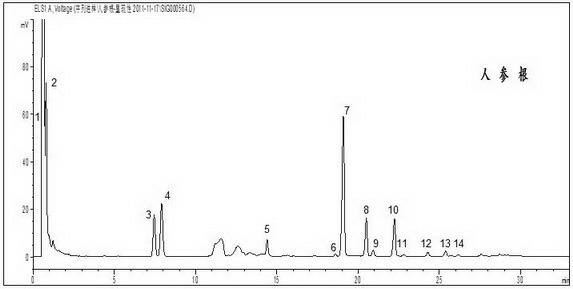
[0111] ginseng root
[0112] 1) Preparation of the test solution: Weigh 2 g of ginseng root to be tested, add 40 ml of chloroform, heat and reflux for 1 hour, discard the chloroform solution, evaporate the dregs of the chloroform, extract with 80 ml of water or methanol or n-butanol , evaporate the solvent, add 30 ml of water to dissolve, add 30 ml of petroleum ether (30~60°C) to extract 1-4 times, 30 ml each time, discard the ether solution, pass the water through a macroporous adsorption resin column, and wash with 50 ml of water ml, 60 ml of 20% ethanol and 70 ml of 75% ethanol were eluted sequentially, the 75% ethanol eluate was collected, evaporated to dryness, the residue was dissolved in methanol, set to volume in a 250 ml volumetric flask, and filtered through a filter membrane to obtain the test product product solution.
[0113] 2) Preparation of reference solution: accurately weigh ginsenoside Rb 1 , dissolved in methanol to make every 1 ml containing ginsenosid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com