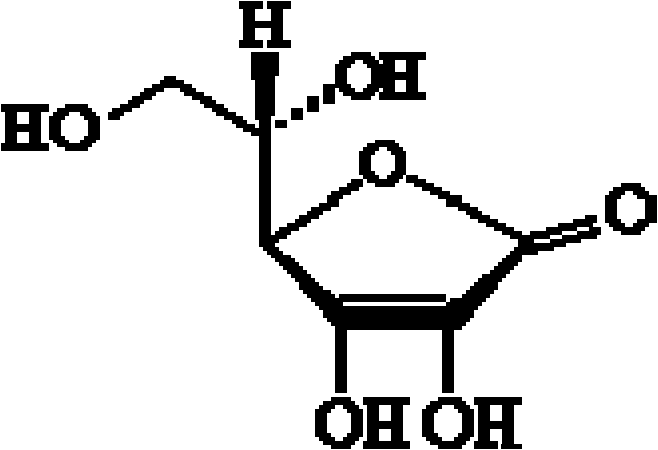
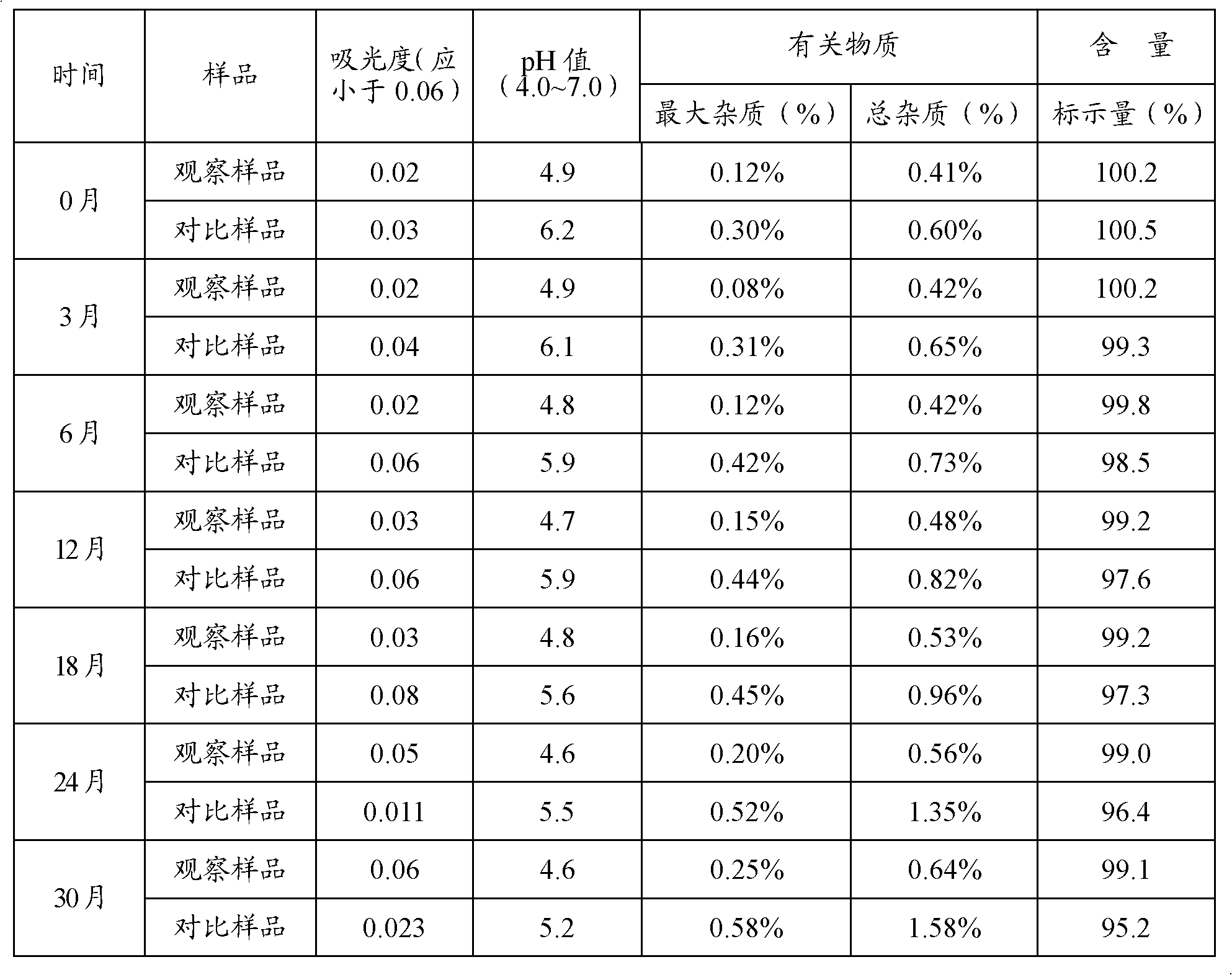
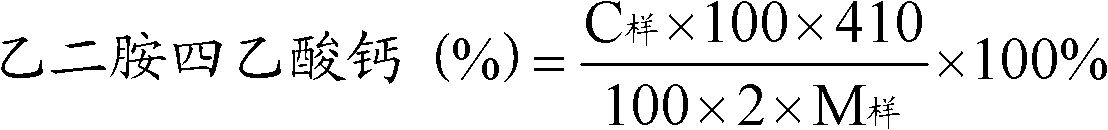Preparation of aseptic vitamin C composition, product and application thereof
A technology for vitamins and compositions, applied in the field of preparation of vitamin C compositions, can solve the problems of difficulty in extraction, complicated production process, increased production cost, etc., and achieves the advantages of reducing process links and material costs, facilitating long-term storage, and avoiding degradation problems. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0054] Add 1 kg of sodium vitamin C to 1 kg of water for injection, then add 10 g of cysteine, 50 g of calcium edetate, and 30 g of activated carbon for needles, and stir at 50±5°C for 20 minutes. Use a 0.22μm filter membrane for sterilizing filtration, inject the filtrate into a sterile crystallization tank, add 3kg of sterile ethanol solution while stirring, and lower the temperature to -4~0°C at a rate of 5°C per hour. In the process, the size of the formed crystals can be appropriately controlled to be 300-500 μm, and then kept at this temperature for 6 hours. Centrifuge and dehydrate the crystallization solution under sterile conditions, wash the crystals with a small amount (about 500g) of acetone, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±2°C, vacuum degree is 0.06-0.08MPa)4 Hours, make its moisture co...
Embodiment 2
[0059] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0060] Add 1 kg of sodium vitamin C to 1.8 kg of water for injection, then add 50 g of cysteine, 20 g of calcium edetate, and 50 g of activated carbon for needles, and stir at 45±5°C for 20 minutes. Use a 0.22 μm filter membrane for sterile filtration, inject the filtrate into a sterile crystallization tank, add 600 g of sterile isopropanol solution while stirring, and lower the temperature to -4 to 0 °C at a rate of 3 °C per hour. During this process, the size of the formed crystals can be appropriately controlled to be 300-500 μm, and then kept at this temperature for 7 hours. Centrifuge and dehydrate the crystallization liquid under sterile conditions, wash the crystals with isopropanol, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±2°C, vacuum degree is 0.06-0.08MPa) for 6 hours. The water content is less tha...
Embodiment 3
[0065] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0066] Add 1 kg of sodium vitamin C to 3 kg of water for injection, then add 20 g of cysteine, 40 g of calcium edetate, and 100 g of activated carbon for needles, and stir for 25 minutes at 55±5°C. Use a 0.22 μm filter membrane for sterile filtration, inject the filtrate into a sterile crystallization tank, add 600 g of sterile isopropanol solution while stirring, and lower the temperature to -4 to 0 °C at a rate of 5 °C per hour. During this process, the size of the formed crystals can be properly controlled to be 300-500 μm, and then kept at this temperature for 8 hours. Centrifuge and dehydrate the crystallization solution under sterile conditions, wash the crystals with isopropanol, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±5°C, vacuum degree is 0.06-0.08MPa) for 8 hours. The water content is less than 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com