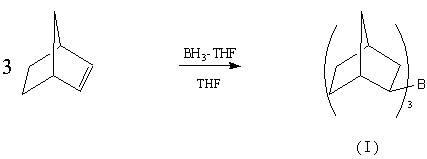
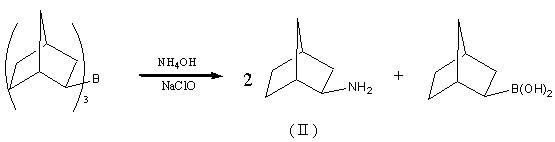
Preparation method of drug intermediate 2-aminonorbornane
A kind of technology of norbornane and borane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) BH 3 · Preparation of THF (borane-tetrahydrofuran adduct)
[0019] Instrument reagent pretreatment:
[0020] All required glass instruments were dried at 110°C and assembled while hot, and cooled to room temperature under nitrogen protection.
[0021] bp: BF at 126℃ / 760mmHg 3 ·Et 2 O is processed as follows: per 500ml BF 3 ·Et 2 Add 2g CaH to O 2 and 10ml of anhydrous diethyl ether, distilled at 70°C under nitrogen protection to obtain a colorless and transparent solution, which should be stored away from light;
[0022] d = 0.9451, bp = 162°C, mp = -68°C Diglyme is treated as follows: CaH 2 Dry and store for 1-2 days until no bubbles overflow, and evaporate at 78°C under reduced pressure.
[0023] Under nitrogen protection, add 148.2g NaBH in the 2000ml three-neck flask in ice-water bath 4 and 1000ml Diglyme (diethylene glycol dimethyl ether), under nitrogen pressure, add BF dropwise through a double-ended needle 3 ·Et 2 O 630ml. Add 2000ml of d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com