Preparation method of cis-3-amino-cyclopentanol hydrochloride
A technology of cyclopentanol hydrochloride and cyclopentanol, which is applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of low purity and potential explosion of cis-3-amino-cyclopentanol Dangerous, low industrialization methods and other problems, to achieve the effect of reducing the difficulty of purification, increasing the yield, and the process is simple and convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
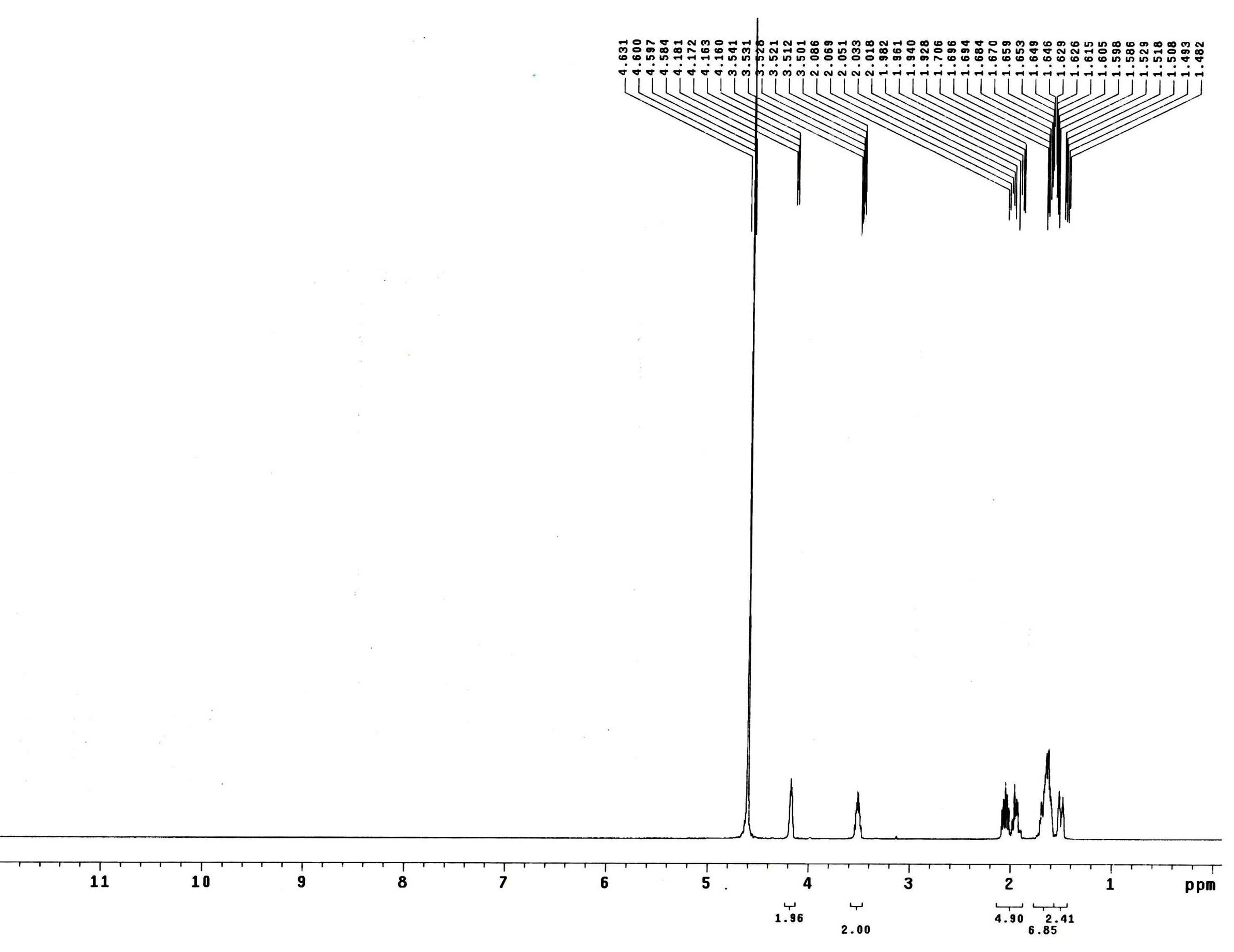
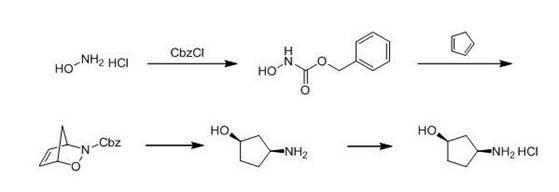
[0031] Take benzyloxycarbonyl chloride and hydroxylamine hydrochloride at a molar ratio of 1:2, dissolve them in dichloromethane, react at room temperature for 16 hours, purify the reaction product, wash the organic phase with saturated brine, and dry at 30°C to 40°C Remove dichloromethane at high temperature, and recrystallize to obtain cis-3-amino-cyclopentanol intermediate A; according to a molar ratio of 3:1, take heavy-distilled cyclopentadiene and cis-3-amino produced in step 1 respectively -Cyclopentanol intermediate A, dissolved in dichloromethane, at a temperature of 0°C to 5°C, add sodium periodate and tetrabutylammonium bromide to react for 6 hours, purify the product, and obtain cis-3 -Amino-cyclopentanol intermediate B; According to the molar ratio of 0.1:1, take the catalyst and cis-3-amino-cyclopentanol intermediate B respectively, dissolve them in methanol, pass hydrogen at room temperature to 50Psi, react for 10 hours, and purify to form cis-3-amino-cyclopenta...
Embodiment 2
[0038] Take benzyloxycarbonyl chloride and hydroxylamine hydrochloride at a molar ratio of 1:2.5, dissolve them in dichloromethane, react at room temperature for 10 hours, purify the reaction product, wash the organic phase with saturated brine, and dry at 30°C to 40°C Remove dichloromethane at high temperature, and recrystallize to obtain cis-3-amino-cyclopentanol intermediate A; according to a molar ratio of 2.2:1, take heavy-distilled cyclopentadiene and cis-3-amino produced in step 1 respectively -Cyclopentanol intermediate A, dissolved in dichloromethane, at a temperature of 0°C to 5°C, add sodium periodate and tetrabutylammonium bromide to react for 4 hours, purify the product, and obtain cis-3 -Amino-cyclopentanol intermediate B; According to the molar ratio of 0.05:1, take the catalyst and cis-3-amino-cyclopentanol intermediate B respectively, dissolve them in methanol, and pass hydrogen at room temperature to 50Psi, react for 10 hours, and purify to form cis-3-amino-c...
Embodiment 3
[0041] Take benzyloxycarbonyl chloride and hydroxylamine hydrochloride at a molar ratio of 1:2.5, dissolve them in dichloromethane, react at room temperature for 16 hours, purify the reaction product, wash the organic phase with saturated brine, and dry at 30°C to 40°C Remove dichloromethane at high temperature, and recrystallize to obtain cis-3-amino-cyclopentanol intermediate A; according to a molar ratio of 4:1, take heavy-distilled cyclopentadiene and cis-3-amino produced in step 1 respectively -Cyclopentanol intermediate A, dissolved in dichloromethane, at a temperature of 0°C to 5°C, add sodium periodate and tetrabutylammonium bromide to react for 6 hours, purify the product, and obtain cis-3 -Amino-cyclopentanol intermediate B; According to the molar ratio of 0.1:1, take the catalyst and cis-3-amino-cyclopentanol intermediate B respectively, dissolve them in methanol, pass hydrogen at room temperature to 50Psi, react for 10 hours, and purify to form cis-3-amino-cyclopen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com