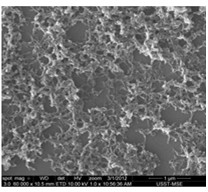
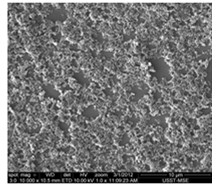
Aminocarbazole/aniline copolymer and synthesis method thereof
A technology of aniline copolymer and aminocarbazole is applied in the field of preparation of novel organic conjugated polymers, which can solve the problems of high price, poor solubility of polyaniline, poor fluorescence performance of polyaniline, etc. Blue-emitting, highly fluorescent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis of Pure Polyaniline Namely Aminocarbazole / Aniline (0 / 100) Copolymer
[0078] The aminocarbazole / aniline copolymerization molar ratio is 0 / 100, that is, accurately weigh 186mg (2mmol) aniline and dissolve it in 13mL ethanol to form a monomer solution;
[0079] According to the molar ratio of oxidizing agent and aniline monomer being 2 / 1, weigh 912 mg (4 mmol) of oxidizing agent ammonium persulfate and dissolve it in 10 mL of 1.0 mol / L hydrochloric acid solution to form an oxidizing agent solution.
[0080] Pour the oxidant solution into the aniline monomer solution, shake vigorously for 10 seconds, control the temperature at 25°C, and let it stand for 24 hours to react. After the reaction is finished, the reaction solution is taken out and centrifugally settled to obtain a solid product, and the product is washed with deionized water; the solid product is washed with 8 times of deionized water, centrifugally settled, and repeated washing-centrifugation three ti...
Embodiment 2
[0083] Synthesis of aminocarbazole / aniline (10 / 90) copolymer
[0084] The molar ratio of aminocarbazole / aniline copolymerization is 10 / 90, that is, accurately weigh 42mg (0.2mmol) of aminocarbazole and 168mg (1.8mmol) of aniline and dissolve them in 13mL of ethanol to form a comonomer solution;
[0085] According to the molar ratio of oxidizing agent and comonomer being 2 / 1, 912 mg (4 mmol) of oxidizing agent ammonium persulfate was weighed and dissolved in 10 mL of 1.0 mol / L hydrochloric acid solution to form an oxidizing agent solution.
[0086] Pour the oxidizing agent solution into the aminocarbazole / aniline comonomer solution, shake vigorously for 10 seconds, control the temperature at 25°C, and let it stand for 24 hours to react. After the reaction is finished, the reaction solution is taken out and centrifugally settled to obtain a solid product, and the product is washed with deionized water; the solid product is washed with 8 times of deionized water, centrifugally se...
Embodiment 3
[0089] Synthesis of aminocarbazole / aniline (30 / 70) copolymer
[0090] The molar ratio of aminocarbazole / aniline copolymerization is 30 / 70, that is, accurately weigh 126mg (0.6mmol) aminocarbazole and 130mg (1.4mmol) aniline and dissolve them in 13mL ethanol to form a comonomer solution;
[0091] According to the molar ratio of oxidizing agent and comonomer being 2 / 1, 912 mg (4 mmol) of oxidizing agent ammonium persulfate was weighed and dissolved in 10 mL of 1.0 mol / L hydrochloric acid solution to form an oxidizing agent solution.
[0092] Pour the oxidizing agent solution into the aminocarbazole / aniline comonomer solution, shake vigorously for 10 seconds, control the temperature at 25°C, and let it stand for 24 hours to react. After the reaction is finished, the reaction solution is taken out and centrifugally settled to obtain a solid product, and the product is washed with deionized water; the solid product is washed with 8 times of deionized water, centrifugally settle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com