Roasting-free evaporation-free method for producing cupric sulfate from zinc hydrometallurgy acid-wash copper dross
A technology for hydro-smelting zinc and copper sulfate, which is applied in the direction of copper sulfate and the improvement of process efficiency, can solve the problems of increased production cost, rising raw material cost, complicated operation, etc., and achieves water consumption saving, fast production speed, and utilization rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0025] Example 1
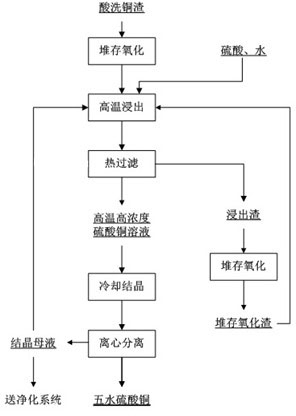
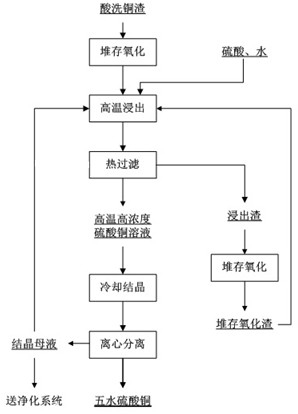
[0026] (1) Store 1.82t of pickled copper slag for 1 day, and rely on the oxygen in the air to make it naturally oxidized; among them, the pickled copper slag is the pickled copper slag produced by the purification of the hydro-zinc smelting solution. The phase is elemental metal copper or copper metal compound, containing copper (total copper) greater than 38.71wt%;
[0027] (2) Using a sulfuric acid solution with a concentration of 250g / L as the leaching agent, leaching the pickled copper slag after oxidation in step (1) at a solid-to-liquid ratio of 1:6 at 100°C for 2.5 hours to obtain a slurry;
[0028] (3) Filter the leached slurry obtained in step (2) at 100°C to obtain the leaching liquid and leaching residue respectively; the obtained leaching residue is piled up to natural oxidation and then returned to step (2) for repeated leaching 5 times until Copper (total copper) in the leaching residue is less than 1wt%;
[0029] (4) The leachate obtained in step (3)...
Example Embodiment
[0031] Example 2
[0032] (1) 2.75t pickling copper slag is stored for 3 days, and it is naturally oxidized by oxygen in the air; among them, the pickling copper slag is the pickling copper slag produced by the purification of the hydro-zinc smelting solution, and the copper content Phase is elemental metal copper or copper metal compound, containing 33.52wt% copper (total copper);
[0033] (2) Using sulfuric acid solution with a concentration of 150g / L as the leaching agent, leaching the pickled copper slag after oxidation in step (1) at 92°C with a solid-to-liquid ratio of 1:5 for 0.5 hours to obtain a slurry;
[0034] (3) Filter the leached slurry obtained in step (2) at 92°C to obtain the leachate and leaching residue respectively; the obtained leaching residue is piled up to natural oxidation and then returned to step (2) for repeated leaching three times until leaching Copper in the slag (total copper) is less than 1wt%;
[0035] (4) The leachate obtained in step (3) is convent...
Example Embodiment
[0037] Example 3
[0038] (1) Pile up 2.79t of pickled copper slag for 5 days, and rely on oxygen in the air to make it naturally oxidized; among them, the pickled copper slag is the pickled copper slag produced by the purification of the hydro-zinc smelting solution. The phase is elemental metal copper or copper metal compound, containing copper (total copper) greater than 34.06wt%;
[0039] (2) Using a sulfuric acid solution with a concentration of 250 g / L as the leaching agent, leaching the pickled copper slag after oxidation in step (1) at a solid-to-liquid ratio of 1:4 at 80°C for 1 hour to obtain a slurry;
[0040] (3) Filter the leached slurry obtained in step (2) at 80°C to obtain leaching liquid and leaching residue respectively; the obtained leaching residue is piled up to natural oxidation and then returned to step (2) for repeated leaching three times until leaching Copper in the slag (total copper) is less than 1wt%;
[0041] (4) The leachate obtained in step (3) is conv...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap