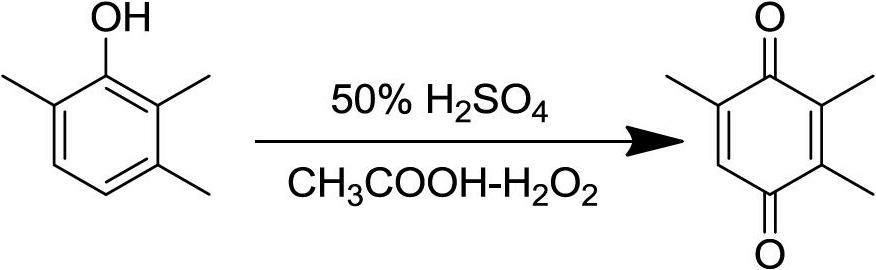Green and simple preparation method for 2,3,5-trimethylbenzoquinone (TMBQ)
A technology of trimethylbenzoquinone and trimethylphenol, which is applied in the field of green and simple preparation of 2,3,5-trimethylbenzoquinone (TMBQ), can solve the problem that the product cannot form a pure crystalline product and is difficult to remove by-products The problems such as product, can not be obtained, etc., to achieve the effect of low reaction equipment requirements, simple and easy implementation of reaction conditions, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
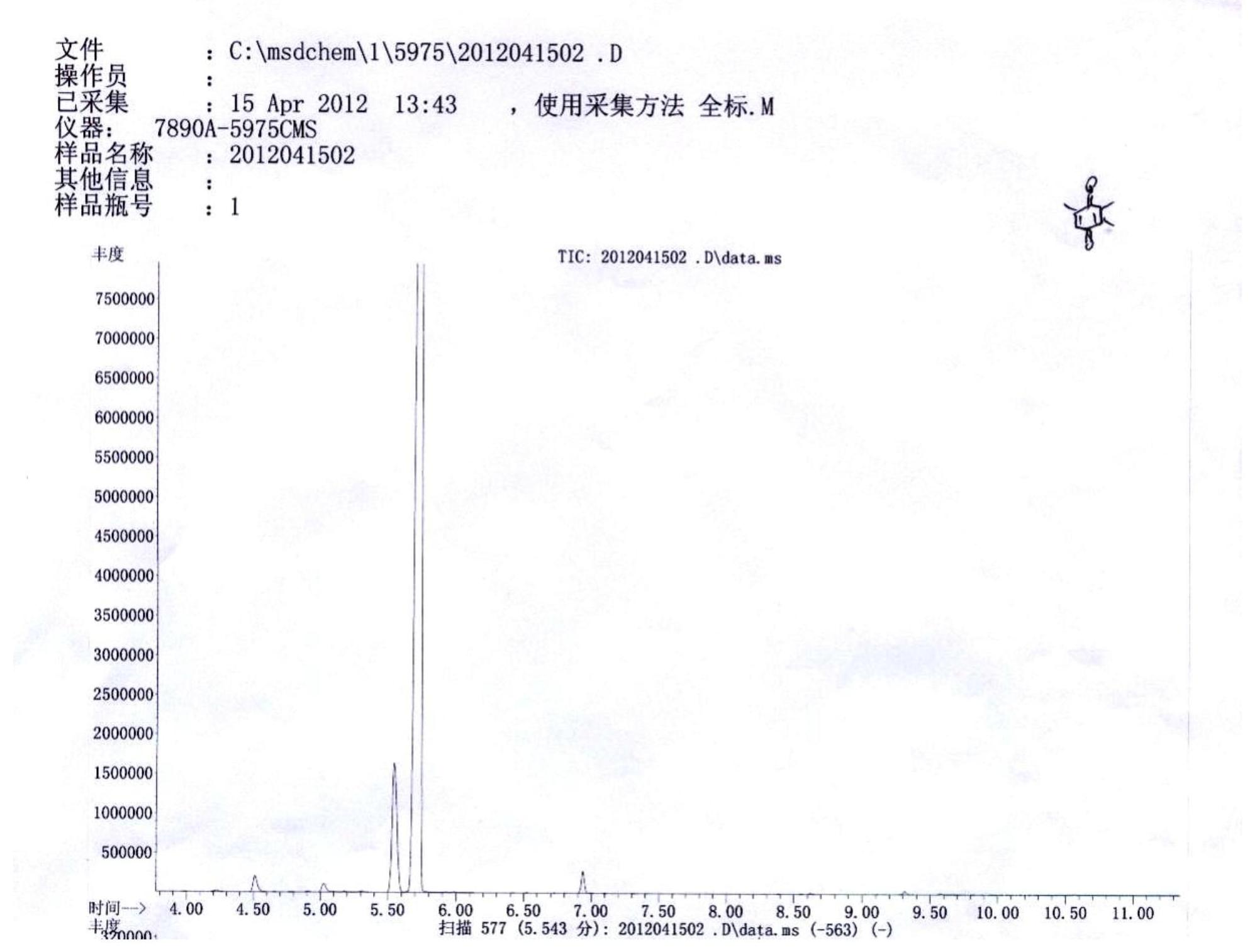
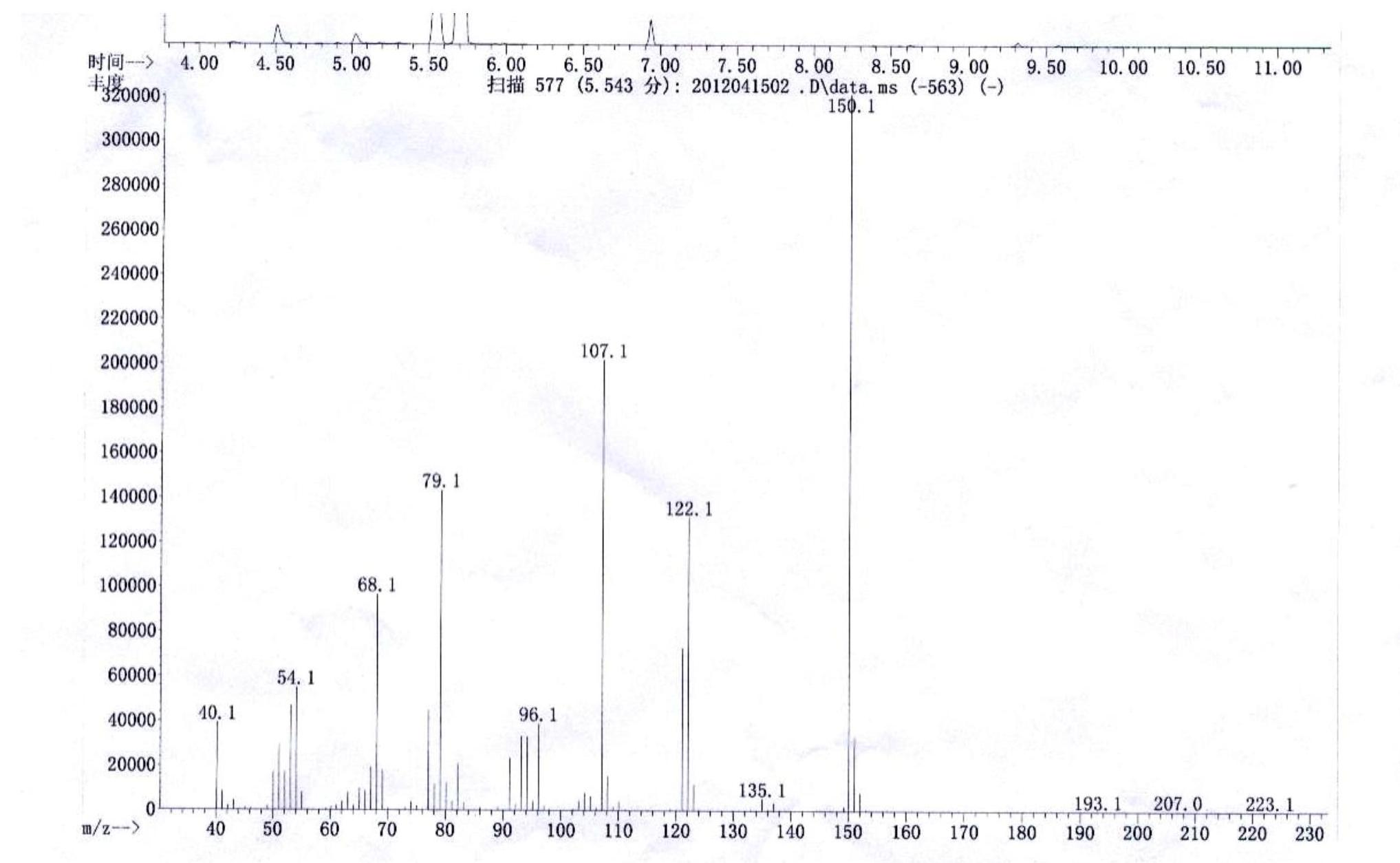
[0037] Weigh 0.03 mol of 2,3,6-trimethylphenol into a 250 mL three-necked flask, add 35 mL of petroleum ether and place it on a constant temperature oil bath with temperature controlled at 30°C. When the raw material is dissolved and the temperature is constant, add 4 times the equivalent of 50% sulfuric acid aqueous solution dropwise. The dropping speed should not be too fast. The dropping speed can be controlled by a constant pressure dropping funnel. After the dropping is completed, keep warm for 30 minutes. Then measure 6 times the equivalent of 30% hydrogen peroxide and add it dropwise to the above reaction system, and react at 30°C for half an hour after the addition is completed. The reaction produces a large amount of earthy matter, after the end, let it stand for stratification, filter to remove the solid, separate the water phase and petroleum ether phase, and then extract the water phase with petroleum ether for 3 times, combine the organic phase, and successively us...
Embodiment 2
[0039] Weigh 0.03 mol of 2,3,6-trimethylphenol into a 250 mL three-necked flask, add 35 mL of petroleum ether and place it on a constant temperature oil bath with temperature controlled at 30°C. When the raw material is dissolved and the temperature is constant, add 4 times the equivalent of 50% sulfuric acid aqueous solution dropwise. The dropping speed should not be too fast. The dropping speed can be controlled by a constant pressure dropping funnel. After the dropping is completed, keep warm for 30 minutes. Measure 6 times the equivalent of acetic acid and 30% hydrogen peroxide (the equivalent of acetic acid and hydrogen peroxide), mix them in a 50mL constant pressure dropping funnel, and add them drop by drop to the above reaction system. React at 30°C for half an hour. After the reaction, the layers were left to stand, and the water phase and petroleum ether phase were separated, and the water phase was extracted with petroleum ether for 3 times, and the organic phase wa...
Embodiment 3
[0041] Weigh 0.03 mol of 2,3,6-trimethylphenol into a 250 mL three-necked flask, add 35 mL of petroleum ether and place it on a constant temperature oil bath with temperature controlled at 30°C. When the raw material is dissolved and the temperature is constant, add 2 times the equivalent of 50% sulfuric acid aqueous solution dropwise. The dropping speed should not be too fast. The dropping speed can be controlled by a constant pressure dropping funnel. After the dropping is completed, keep warm for 30 minutes. Measure 6 times the equivalent of acetic acid and 30% hydrogen peroxide (the equivalent of acetic acid and hydrogen peroxide), mix them in a 50mL constant pressure dropping funnel, and add them drop by drop to the above reaction system. React at 30°C for half an hour. After the reaction, the layers were left to stand, and the water phase and petroleum ether phase were separated, and the water phase was extracted with petroleum ether for 3 times, and the organic phase wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com